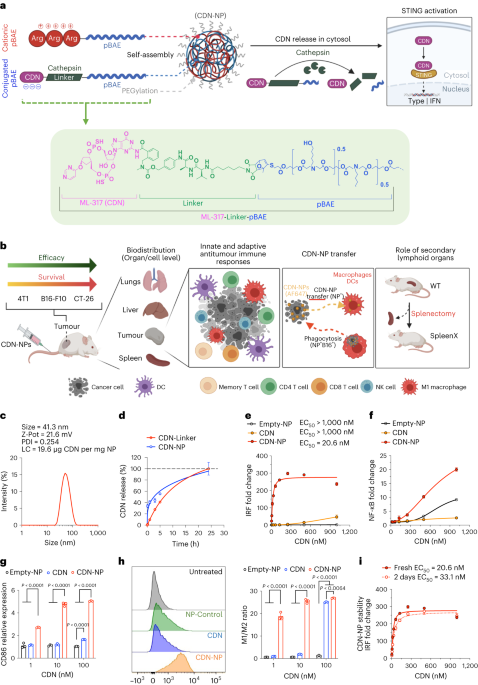
Investigation of the enhanced antitumour potency of sting agonist after conjugation to polymer nanoparticles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Intravenously administered cyclic dinucleotides and other STING agonists are hampered by low cellular uptake and poor circulatory half-life. Here we report the covalent conjugation
of cyclic dinucleotides to poly(β-amino ester) nanoparticles through a cathepsin-sensitive linker. This is shown to increase stability and loading, thereby expanding the therapeutic window
in multiple syngeneic tumour models, enabling the study of how the long-term fate of the nanoparticles affects the immune response. In a melanoma mouse model, primary tumour clearance
depends on the STING signalling by host cells—rather than cancer cells—and immune memory depends on the spleen. The cancer cells act as a depot for the nanoparticles, releasing them over
time to activate nearby immune cells to control tumour growth. Collectively, this work highlights the importance of nanoparticle structure and nano-biointeractions in controlling
immunotherapy efficacy. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS UNIVERSAL STING MIMIC BOOSTS ANTITUMOUR IMMUNITY VIA PREFERENTIAL ACTIVATION OF TUMOUR CONTROL SIGNALLING PATHWAYS Article 13 March 2024 CHEMICALLY PROGRAMMED
STING-ACTIVATING NANO-LIPOSOMAL VESICLES IMPROVE ANTICANCER IMMUNITY Article Open access 31 July 2023 ZINC CYCLIC DI-AMP NANOPARTICLES TARGET AND SUPPRESS TUMOURS VIA ENDOTHELIAL STING
ACTIVATION AND TUMOUR-ASSOCIATED MACROPHAGE REINVIGORATION Article 27 October 2022 DATA AVAILABILITY All data generated or analysed that support the findings of this study are available
within this Article and its Supplementary Information. All raw data from this study are available from the corresponding author upon request. Source data are provided with this paper.
REFERENCES * Yum, S., Li, M., Frankel, A. E. & Chen, Z. J. Roles of the cGAS-STING pathway in cancer immunosurveillance and immunotherapy. _Annu. Rev. Cancer Biol._ 3, 323–344 (2019).
Google Scholar * Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS–STING pathway in cancer. _Cancer Discov._ 10, 26 (2020). CAS Google Scholar * Ishikawa, H., Ma, Z. &
Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. _Nature_ 461, 788–792 (2009). CAS Google Scholar * Barber, G. N. STING: infection,
inflammation and cancer. _Nat. Rev. Immunol._ 15, 760–770 (2015). CAS Google Scholar * Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer
immunity. _Nat. Rev. Immunol._ 15, 405–414 (2015). CAS Google Scholar * Woo, S.-R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors.
_Immunity_ 41, 830–842 (2014). CAS Google Scholar * Nicolai, C. J. et al. NK cells mediate clearance of CD8+ T cell–resistant tumors in response to STING agonists. _Sci. Immunol._ 5,
eaaz2738 (2020). * Nakamura, T. et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. _J. Immunother. Cancer_ 9,
e002852 (2021). * Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. _Sci. Transl. Med._ 7, 283ra252 (2015). Google Scholar *
Dosta, P. et al. Delivery of stimulator of interferon genes (STING) agonist using polypeptide-modified dendrimer nanoparticles in the treatment of melanoma. _Adv. NanoBiomed Res._ 1, 2100006
(2021). CAS Google Scholar * Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. _Nature_ 498, 380–384 (2013). CAS Google Scholar
* Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. _Science_ 339, 826–830 (2013). Google Scholar * Zhang, X. et al. Cyclic
GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. _Mol. Cell_ 51, 226–235 (2013). CAS Google Scholar * Lee, S. E. et al. Improvement of
STING-mediated cancer immunotherapy using immune checkpoint inhibitors as a game-changer. _Cancer Immunol. Immunother._ 71, 3029–3042 (2022). CAS Google Scholar * Jneid, B. et al.
Selective STING stimulation in dendritic cells primes antitumor T cell responses. _Sci. Immunol._ 8, eabn6612 (2023). CAS Google Scholar * Wang, H. et al. cGAS is essential for the
antitumor effect of immune checkpoint blockade. _Proc. Natl Acad. Sci. USA_ 114, 1637–1642 (2017). CAS Google Scholar * Meric-Bernstam, F. et al. Phase Ib study of MIW815 (ADU-S100) in
combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. _J. Clin. Oncol._ 37, 2507 (2019). Google Scholar * Harrington, K. J. et al.
Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in
patients with advanced solid tumors or lymphomas. _Ann. Oncol._ 29, VIII712 (2018). Google Scholar * Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide
STING agonists to enhance cancer immunotherapy. _Nat. Nanotechnol._ 14, 269–278 (2019). CAS Google Scholar * Watkins-Schulz, R. et al. A microparticle platform for STING-targeted
immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity. _Biomaterials_ 205, 94–105 (2019). CAS Google Scholar * Koshy, S. T., Cheung, A. S., Gu, L.,
Graveline, A. R. & Mooney, D. J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. _Adv. Biosyst._ 1, 1600013 (2017). Google Scholar * Lin, Z. P.
et al. Macrophages actively transport nanoparticles in tumors after extravasation. _ACS Nano_ 16, 6080–6092 (2022). CAS Google Scholar * Miller, M. A. et al. Tumour-associated macrophages
act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. _Nat. Commun._ 6, 8692 (2015). CAS Google Scholar * Korangath, P. et al. Nanoparticle interactions with immune cells
dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. _Sci. Adv._ 6, eaay1601 (2020). CAS Google Scholar * Dane, E. L. et al. STING agonist
delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. _Nat. Mater._ 21, 710–720 (2022). CAS Google Scholar * Sun, X. et al. Amplifying STING activation by
cyclic dinucleotide–manganese particles for local and systemic cancer metalloimmunotherapy. _Nat. Nanotechnol._ 16, 1260–1270 (2021). CAS Google Scholar * Riley, R. S., June, C. H.,
Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. _Nat. Rev. Drug Discov._ 18, 175–196 (2019). CAS Google Scholar * Wehbe, M. et al. Nanoparticle delivery
improves the pharmacokinetic properties of cyclic dinucleotide STING agonists to open a therapeutic window for intravenous administration. _J. Control. Release_ 330, 1118–1129 (2021). CAS
Google Scholar * Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. _Sci. Immunol._ 4, eaau6085 (2019). * Bronte, V. & Pittet,
MikaelJ. The spleen in local and systemic regulation of immunity. _Immunity_ 39, 806–818 (2013). CAS Google Scholar * Segovia, N., Dosta, P., Cascante, A., Ramos, V. & Borrós, S.
Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene delivery and intracellular localization. _Acta Biomater._ 10, 2147–2158 (2014). CAS Google Scholar * Dosta, P.,
Ramos, V. & Borrós, S. Stable and efficient generation of poly(β-amino ester)s for RNAi delivery. _Mol. Syst. Des. Eng._ 3, 677–689 (2018). CAS Google Scholar * Dosta, P. et al.
Delivery of anti-microRNA-712 to inflamed endothelial cells using poly(beta-amino ester) nanoparticles conjugated with VCAM-1 targeting peptide. _Adv. Healthcare Mater._ 10, 2001894 (2021).
* Nunez-Toldra, R. et al. Improvement of osteogenesis in dental pulp pluripotent-like stem cells by oligopeptide-modified poly(beta-amino ester)s. _Acta Biomater._ 53, 152–164 (2017). CAS
Google Scholar * Dosta, P. et al. Delivery of siRNA to endothelial cells in vivo using lysine/histidine oligopeptide-modified poly(beta-amino ester) nanoparticles. _Cardiovasc. Eng.
Technol._ 12, 114–125 (2021). Google Scholar * Dosta, P., Segovia, N., Cascante, A., Ramos, V. & Borrós, S. Surface charge tunability as a powerful strategy to control electrostatic
interaction for high efficiency silencing, using tailored oligopeptide-modified poly(beta-amino ester)s (PBAEs). _Acta Biomater._ 20, 82–93 (2015). CAS Google Scholar * Puigmal, N., Ramos,
V., Artzi, N. & Borrós, S. Poly(β-amino ester)s-based delivery systems for targeted transdermal vaccination. _Pharmaceutics_ 15, 1262 (2023). CAS Google Scholar * Vyskocil, S. et al.
Identification of novel carbocyclic pyrimidine cyclic dinucleotide STING agonists for antitumor immunotherapy using systemic intravenous route. _J. Med. Chem._ 64, 6902–6923 (2021). CAS
Google Scholar * Alouane, A., Labruère, R., Le Saux, T., Schmidt, F. & Jullien, L. Self-immolative spacers: kinetic aspects, structure–property relationships, and applications. _Angew.
Chem. Int. Ed._ 54, 7492–7509 (2015). CAS Google Scholar * Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody–drug conjugates. _Chem. Soc.
Rev._ 48, 4361–4374 (2019). CAS Google Scholar * Gandini, A. The furan/maleimide Diels–Alder reaction: a versatile click–unclick tool in macromolecular synthesis. _Prog. Polym. Sci._ 38,
1–29 (2013). CAS Google Scholar * Froidevaux, V. et al. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials.
_RSC Adv._ 5, 37742–37754 (2015). CAS Google Scholar * Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. _Nat. Rev. Drug Discov._ 2, 214–221 (2003). CAS Google
Scholar * Fornaguera, C. et al. mRNA delivery system for targeting antigen-presenting cells in vivo. _Adv. Healthcare Mater._ 7, 1800335 (2018). Google Scholar * Blanco, E., Shen, H. &
Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. _Nat. Biotechnol._ 33, 941–951 (2015). CAS Google Scholar * Cheng, Q. et al. Selective
organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. _Nat. Nanotechnol._ 15, 313–320 (2020). * Akdis, C. A. & Blaser, K. Mechanisms of
interleukin-10-mediated immune suppression. _Immunology_ 103, 131–136 (2001). CAS Google Scholar * Brown, M. A. & Hural, J. Functions of IL-4 and control of its expression. _Crit. Rev.
Immunol._ 17, 1–32 (1997). CAS Google Scholar * Goswami, R. & Kaplan, M. H. A brief history of IL-9. _J. Immunol._ 186, 3283 (2011). CAS Google Scholar * Harlin, H. et al. Chemokine
expression in melanoma metastases associated with CD8+ T-cell recruitment. _Cancer Res._ 69, 3077–3085 (2009). Google Scholar * Sivick, K. E. et al. Magnitude of therapeutic STING
activation determines CD8+ T cell-mediated anti-tumor immunity. _Cell Rep._ 25, 3074–3085.e3075 (2018). CAS Google Scholar * Lechner, M. G. et al. Immunogenicity of murine solid tumor
models as a defining feature of in vivo behavior and response to immunotherapy. _J. Immunother._ 36, 477–489 (2013). CAS Google Scholar * Fitzgerald-Bocarsly, P., Dai, J. & Singh, S.
Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. _Cytokine Growth Factor Rev._ 19, 3–19 (2008). CAS Google Scholar * Liang, H. et al. Host STING-dependent MDSC
mobilization drives extrinsic radiation resistance. _Nat. Commun._ 8, 1736 (2017). Google Scholar * Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy.
_Cell_ 168, 487–502.e415 (2017). CAS Google Scholar * Poncette, L., Bluhm, J. & Blankenstein, T. The role of CD4 T cells in rejection of solid tumors. _Curr. Opin. Immunol._ 74, 18–24
(2022). CAS Google Scholar * Schadt, L. et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. _Cell Rep._ 29, 1236–1248.e1237 (2019). CAS Google Scholar * Carozza,
J. A. et al. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. _Nat. Cancer_ 1, 184–196 (2020). CAS Google Scholar *
Madaan, A., Verma, R., Singh, A. T., Jain, S. K. & Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. _J. Biol. Methods_ 1, e1 (2014).
Google Scholar Download references ACKNOWLEDGEMENTS We are grateful for the support and funding of Takeda Pharmaceuticals. We thank the Hale Building for Transformative Medicine, the Koch
Institute for Integrative Cancer Research at the Massachusetts Institute of Technology (MIT) and at Takeda Boston for the assistance with animal housing. We also thank A. M. Hayward and P.
Chamberlain from the Department of Comparative Medicine at MIT for animal assistance, G. Paradis for FACS assistance with Cancer Center Support (FACS core) and K. Cormier for histology
assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, USA Pere Dosta, Alexander M.
Cryer, Michelle Z. Dion, Núria Puigmal, Shiran Ferber, Santhosh Kalash, Michaela Prado, Alma L. Rodríguez & Natalie Artzi * Department of Medicine, Division of Engineering in Medicine,
Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA Pere Dosta, Alexander M. Cryer, Michelle Z. Dion, Núria Puigmal, Shiran Ferber, Santhosh Kalash, Michaela Prado, Alma L.
Rodríguez & Natalie Artzi * Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA, USA Pere Dosta, Alexander M. Cryer, Michelle Z. Dion, Núria Puigmal
& Natalie Artzi * Harvard-MIT Division of Health Sciences & Technology, Massachusetts Institute of Technology, Cambridge, MA, USA Michelle Z. Dion * Takeda Development Center
Americas, Inc. (TDCA), Lexington, MA, USA Tsubasa Shiraishi, Steven P. Langston, David Lok, Jianing Wang, Sean Harrison, Tiquella Hatten, Michelle L. Ganno, Vicky A. Appleman, Walid S.
Kamoun & Adnan O. Abu-Yousif * Biodevek, Cambridge, MA, USA Gonzalo Muñoz Taboada Authors * Pere Dosta View author publications You can also search for this author inPubMed Google
Scholar * Alexander M. Cryer View author publications You can also search for this author inPubMed Google Scholar * Michelle Z. Dion View author publications You can also search for this
author inPubMed Google Scholar * Tsubasa Shiraishi View author publications You can also search for this author inPubMed Google Scholar * Steven P. Langston View author publications You can
also search for this author inPubMed Google Scholar * David Lok View author publications You can also search for this author inPubMed Google Scholar * Jianing Wang View author publications
You can also search for this author inPubMed Google Scholar * Sean Harrison View author publications You can also search for this author inPubMed Google Scholar * Tiquella Hatten View author
publications You can also search for this author inPubMed Google Scholar * Michelle L. Ganno View author publications You can also search for this author inPubMed Google Scholar * Vicky A.
Appleman View author publications You can also search for this author inPubMed Google Scholar * Gonzalo Muñoz Taboada View author publications You can also search for this author inPubMed
Google Scholar * Núria Puigmal View author publications You can also search for this author inPubMed Google Scholar * Shiran Ferber View author publications You can also search for this
author inPubMed Google Scholar * Santhosh Kalash View author publications You can also search for this author inPubMed Google Scholar * Michaela Prado View author publications You can also
search for this author inPubMed Google Scholar * Alma L. Rodríguez View author publications You can also search for this author inPubMed Google Scholar * Walid S. Kamoun View author
publications You can also search for this author inPubMed Google Scholar * Adnan O. Abu-Yousif View author publications You can also search for this author inPubMed Google Scholar * Natalie
Artzi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.D., A.O.A.-Y. and N.A. conceived the study. P.D. designed the experiments. S.P.L.,
D.L., J.W. and S.H. performed the ML-317 synthesis and quantification. P.D., A.M.C., M.Z.D., D.L., J.W., S.H., G.M.T., N.P., S.F., M.P. and A.L.R. performed the in vitro experiments. P.D.,
A.M.C., S.K., T.H., M.L.G. and V.A.A. performed the in vivo experiments. P.D., A.M.C., M.Z.D. and N.A. drafted and finalized the manuscript with input from all other authors. CORRESPONDING
AUTHORS Correspondence to Pere Dosta or Natalie Artzi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Nanotechnology_ thanks Jordan Green, Jason Luke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–51,
discussion and uncropped scans of all blots and gels. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Raw data. SOURCE DATA FIG. 2 Raw data. SOURCE DATA FIG. 3 Raw data. SOURCE DATA FIG. 4
Raw data. SOURCE DATA FIG. 5 Raw data. SOURCE DATA FIG. 6 Raw data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this
article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of
such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dosta, P., Cryer, A.M., Dion, M.Z. _et al._ Investigation of the enhanced
antitumour potency of STING agonist after conjugation to polymer nanoparticles. _Nat. Nanotechnol._ 18, 1351–1363 (2023). https://doi.org/10.1038/s41565-023-01447-7 Download citation *
Received: 20 December 2021 * Accepted: 31 May 2023 * Published: 13 July 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41565-023-01447-7 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
