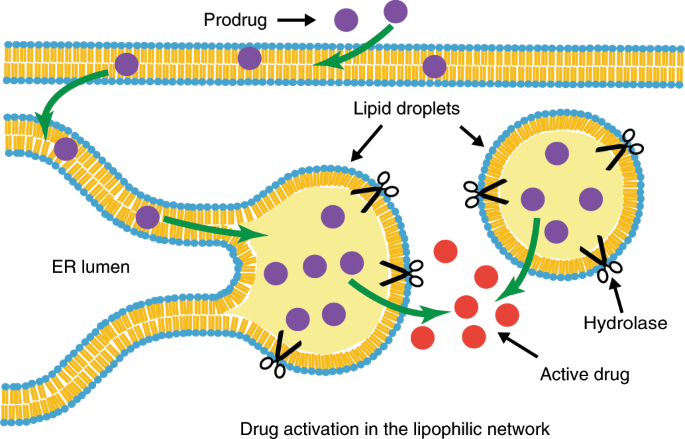
Lipid droplets can promote drug accumulation and activation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Genetic screens in cultured human cells represent a powerful unbiased strategy to identify cellular pathways that determine drug efficacy, providing critical information for
clinical development. We used insertional mutagenesis-based screens in haploid cells to identify genes required for the sensitivity to lasonolide A (LasA), a macrolide derived from a marine
sponge that kills certain types of cancer cells at low nanomolar concentrations. Our screens converged on a single gene, _LDAH_, encoding a member of the metabolite serine hydrolase family
that is localized on the surface of lipid droplets. Mechanistic studies revealed that LasA accumulates in lipid droplets, where it is cleaved into a toxic metabolite by LDAH. We suggest that
selective partitioning of hydrophobic drugs into the oil phase of lipid droplets can influence their activation and eventual toxicity to cells. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only
$21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS GENETIC AND PHARMACOLOGICAL
INTERROGATION OF CANCER VULNERABILITY USING A MULTIPLEXED CELL LINE SCREENING PLATFORM Article Open access 02 July 2021 METABOLIC DRUG SURVEY HIGHLIGHTS CANCER CELL DEPENDENCIES AND
VULNERABILITIES Article Open access 14 December 2021 SYNTHETIC LETHAL STRATEGIES FOR THE DEVELOPMENT OF CANCER THERAPEUTICS Article 03 December 2024 DATA AVAILABILITY The complete lists of
the hits from the genetic screens are given in Supplementary Data 1. RNA-seq data from Hap1 cells is freely available at NCBI GEO, under accession no. GSE75515. The GI50 data for LasA and
the RNA-seq data for cancer cell lines is publicly available (accession numbers given in the appropriate Methods section). Software for analysis of screen results has been described
previously9,10 and is freely available on github: https://github.com/RohatgiLab/BAIMS-Pipeline. REFERENCES * Horton, P. A., Koehn, F. E., Longley, R. E. & McConnell, O. J. Lasonolide A,
a new cytotoxic macrolide from the marine sponge _Forcepia_ sp. _J. Am. Chem. Soc._ 116, 6015–6016 (1994). Article CAS Google Scholar * Wright, A. E. et al. Lasonolides C–G, five new
lasonolide compounds from the sponge _Forcepia_ sp. _J. Nat. Prod._ 67, 1351–1355 (2004). Article CAS Google Scholar * Isbrucker, R. A., Guzman, E. A., Pitts, T. P. & Wright, A. E.
Early effects of lasonolide A on pancreatic cancer cells. _J. Pharmacol. Exp. Ther._ 331, 733–739 (2009). Article CAS Google Scholar * Zhang, Y. W., Ghosh, A. K. & Pommier, Y.
Lasonolide A, a potent and reversible inducer of chromosome condensation. _Cell Cycle_ 11, 4424–4435 (2012). Article CAS Google Scholar * Josse, R. et al. Activation of RAF1 (c-RAF) by
the marine alkaloid lasonolide A induces rapid premature chromosome condensation. _Mar. Drugs_ 13, 3625–3639 (2015). Article CAS Google Scholar * Trost, B. M. et al. Total synthesis of
(−)-lasonolide A. _J. Am. Chem. Soc._ 138, 11690–11701 (2016). Article CAS Google Scholar * Carette, J. E. et al. Haploid genetic screens in human cells identify host factors used by
pathogens. _Science_ 326, 1231–1235 (2009). Article CAS Google Scholar * Carette, J. E. et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing.
_Nat. Biotechnol._ 29, 542–546 (2011). Article CAS Google Scholar * Dubey, R. et al. Chromatin-remodeling complex SWI/SNF controls multidrug resistance by transcriptionally regulating the
drug efflux pump ABCB1. _Cancer Res._ 76, 5810–5821 (2016). Article CAS Google Scholar * Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1.
_Nature_ 477, 340–343 (2011). Article CAS Google Scholar * Simon, G. M. & Cravatt, B. F. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. _J.
Biol. Chem._ 285, 11051–11055 (2010). Article CAS Google Scholar * Bachovchin, D. A. & Cravatt, B. F. The pharmacological landscape and therapeutic potential of serine hydrolases.
_Nat. Rev. Drug Discov._ 11, 52–68 (2012). Article CAS Google Scholar * Hebenstreit, D. et al. RNA sequencing reveals two major classes of gene expression levels in metazoan cells. _Mol.
Syst. Biol._ 7, 497–497 (2011). Article Google Scholar * Goo, Y. H., Son, S. H., Kreienberg, P. B. & Paul, A. Novel lipid droplet-associated serine hydrolase regulates macrophage
cholesterol mobilization. _Arterioscler. Thromb. Vasc. Biol._ 34, 386–396 (2014). Article CAS Google Scholar * Thiel, K. et al. The evolutionarily conserved protein CG9186 is associated
with lipid droplets, required for their positioning and for fat storage. _J. Cell Sci._ 126, 2198–2212 (2013). Article CAS Google Scholar * Marchler-Bauer, A. et al. CDD: NCBI’s conserved
domain database. _Nucleic Acids Res._ 43, D222–D226 (2015). Article CAS Google Scholar * Currall, B. B. et al. Loss of LDAH associated with prostate cancer and hearing loss. _Hum. Mol.
Genet._ 27, 4194–4203 (2018). Article CAS Google Scholar * Kory, N. et al. Mice lacking lipid droplet-associated hydrolase, a gene linked to human prostate cancer, have normal cholesterol
ester metabolism. _J. Lipid Res._ 58, 226–235 (2017). Article CAS Google Scholar * Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. _Nat. Rev. Mol. Cell Biol._
20, 137–155 (2019). Article CAS Google Scholar * Brasaemle, D. L. & Wolins, N. E. Isolation of lipid droplets from cells by density gradient centrifugation. _Curr. Protoc. Cell
Biol._ 72, 3.15.1–3.15.13 (2016). Article Google Scholar * Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. _Nat.
Rev. Mol. Cell Biol._ 18, 285–298 (2017). Article CAS Google Scholar * Greenwood, D. J. et al. Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected
macrophages. _Science_ 364, 1279–1282 (2019). Article CAS Google Scholar * Zheng, N., Tsai, H. N., Zhang, X. & Rosania, G. R. The subcellular distribution of small molecules: from
pharmacokinetics to synthetic biology. _Mol. Pharm._ 8, 1619–1628 (2011). Article CAS Google Scholar * den Brok, M. H., Raaijmakers, T. K., Collado-Camps, E. & Adema, G. J. Lipid
droplets as immune modulators in myeloid cells. _Trends Immunol._ 39, 380–392 (2018). Article Google Scholar * Fowler, S., Shio, H. & Haley, N. J. Characterization of lipid-laden
aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. _Lab. Investig._ 41, 372–378 (1979). CAS PubMed Google Scholar *
Foley, P. Lipids in Alzheimer’s disease: a century-old story. _Biochim. Biophys. Acta_ 1801, 750–753 (2010). Article CAS Google Scholar * Delikatny, E. J., Chawla, S., Leung, D.-J. &
Poptani, H. MR-visible lipids and the tumor microenvironment. _NMR Biomedicine_ 24, 592–611 (2011). CAS Google Scholar * Petan, T., Jarc, E. & Jusović, M. Lipid droplets in cancer:
guardians of fat in a stressful world. _Molecules_ 23, 1941 (2018). Article Google Scholar * Sundelin, J. P. et al. Increased expression of the very low-density lipoprotein receptor
mediates lipid accumulation in clear-cell renal cell carcinoma. _PLoS ONE_ 7, e48694 (2012). Article CAS Google Scholar * Hager, M. H., Solomon, K. R. & Freeman, M. R. The role of
cholesterol in prostate cancer. _Curr. Opin. Clin. Nutr. Metab. Care_ 9, 379–385 (2006). Article CAS Google Scholar * Aboumrad, M. H., Horn, R. C. Jr. & Fine, G. Lipid-secreting
mammary carcinoma. Report of a case associated with Paget’s disease of the nipple. _Cancer_ 16, 521–525 (1963). Article CAS Google Scholar * Ramos, C. V. & Taylor, H. B. Lipid-rich
carcinoma of the breast. A clinicopathologic analysis of 13 examples. _Cancer_ 33, 812–819 (1974). Article CAS Google Scholar * Rautio, J., Meanwell, N. A., Di, L. & Hageman, M. J.
The expanding role of prodrugs in contemporary drug design and development. _Nat. Rev. Drug Discov._ 17, 559–587 (2018). Article CAS Google Scholar * Ran, F. A. et al. Genome engineering
using the CRISPR-Cas9 system. _Nat. Protoc._ 8, 2281–2308 (2013). Article CAS Google Scholar * Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for
CRISPR screening. _Nat. Methods_ 11, 783–784 (2014). Article CAS Google Scholar * Campeau, E. et al. A versatile viral system for expression and depletion of proteins in mammalian cells.
_PLoS ONE_ 4, e6529 (2009). Article Google Scholar * Trost, B. M. et al. A concise synthesis of (−)-lasonolide A. _J. Am. Chem. Soc._ 136, 88–91 (2014). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We thank D. Herschlag for bringing the LasA project to our attention, C. Pataki and R. Kopito for comments and advice on lipid droplet fractionation
experiments and A. Lebensohn for advice on the project. The work was funded by DP2 GM105448 (R.R.), R35 GM118082 (R.R.), DP2 AI104557 (J.E.C.), American Heart Association Transformational
Research Projects no. 18TPA34230103 (A.P.) and no. 18TPA34230086 (Y.-H.G.), and Dominic Ferraioli Foundation (A.P.). R.R. is a Josephine Q. Berry Faculty Scholar in Cancer Research at
Stanford, J.E.C. is a David and Lucile Packard Foundation fellow and R.D. was supported by fellowships from the Stanford Dean’s Fund and Alex’s Lemonade Stand Foundation. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Biochemistry, Stanford University School of Medicine, Stanford, CA, USA Ramin Dubey & Rajat Rohatgi * Department of Chemistry, Stanford
University, Stanford, CA, USA Craig E. Stivala & Barry M. Trost * Genentech, South San Francisco, CA, USA Craig E. Stivala & Huy Quoc Nguyen * Department of Molecular and Cellular
Physiology, Albany Medical College, Albany, NY, USA Young-Hwa Goo & Antoni Paul * Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA Jan
E. Carette * Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA Rajat Rohatgi Authors * Ramin Dubey View author publications You can also search for this
author inPubMed Google Scholar * Craig E. Stivala View author publications You can also search for this author inPubMed Google Scholar * Huy Quoc Nguyen View author publications You can also
search for this author inPubMed Google Scholar * Young-Hwa Goo View author publications You can also search for this author inPubMed Google Scholar * Antoni Paul View author publications
You can also search for this author inPubMed Google Scholar * Jan E. Carette View author publications You can also search for this author inPubMed Google Scholar * Barry M. Trost View author
publications You can also search for this author inPubMed Google Scholar * Rajat Rohatgi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
R.R. and R.D. designed the project. B.M.T. and C.E.S. designed and synthesized LasA, LasF, Ces-73, Ces-24a and Ces-24b. R.D. and J.E.C. executed the haploid genetic screens. R.D. and H.Q.N.
performed the mass spectrometry experiments. R.D., A.P. and Y.-H.G. designed and constructed the LDAH variants. R.D. performed all other experiments and analyses presented in the paper. R.R.
and R.D. wrote the paper and all the authors edited and commented on the paper. CORRESPONDING AUTHOR Correspondence to Rajat Rohatgi. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–12. REPORTING SUMMARY SUPPLEMENTARY VIDEO 1 Movie of live Hap1 cells expressing LDAH-GFP. SUPPLEMENTARY DATA 1
Compiled data from the haploid screens (see Fig. 1c and Supplementary Figs. 1 and 2a). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dubey, R.,
Stivala, C.E., Nguyen, H.Q. _et al._ Lipid droplets can promote drug accumulation and activation. _Nat Chem Biol_ 16, 206–213 (2020). https://doi.org/10.1038/s41589-019-0447-7 Download
citation * Received: 13 February 2019 * Accepted: 02 December 2019 * Published: 13 January 2020 * Issue Date: February 2020 * DOI: https://doi.org/10.1038/s41589-019-0447-7 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
