Pleistocene allopatric differentiation followed by recent range expansion explains the distribution and molecular diversity of two congeneric crustacean species in the palaearctic
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Pleistocene glaciations had a tremendous impact on the biota across the Palaearctic, resulting in strong phylogeographic signals of range contraction and rapid postglacial
recolonization of the deglaciated areas. Here, we explore the diversity patterns and history of two sibling species of passively dispersing taxa typical of temporary ponds, fairy shrimps
(Anostraca). We combine mitochondrial (COI) and nuclear (ITS2 and 18S) markers to conduct a range-wide phylogeographic study including 56 populations of _Branchinecta ferox_ and
_Branchinecta orientalis_ in the Palaearctic. Specifically, we investigate whether their largely overlapping ranges in Europe resulted from allopatric differentiation in separate glacial
refugia followed by a secondary contact and reconstruct their postglacial recolonization from the inhabited refugia. Our results suggest the existence of distinct refugia for the two
species, with genetic divergence among intraspecific lineages consistent with late Pleistocene glacial cycles. While _B. ferox_ lineages originated from Mediterranean refugia, the origin of
_B. orientalis_ lineages was possibly located on the Pannonian Plain. We showed that most dispersal events predominantly happened within 100 km, coupled with several recent long-distance
events (> 1000 km). Hence the regional habitat density of suitable habitats in Central Europe is possibly a key to the co-existence of the two species. Overall, our study illustrates how
isolation in combination with stochastic effects linked to glacial periods are important drivers of the allopatric differentiation of Palaearctic taxa. SIMILAR CONTENT BEING VIEWED BY OTHERS
LONG-TERM CLIMATIC STABILITY DRIVES ACCUMULATION AND MAINTENANCE OF DIVERGENT FRESHWATER FISH LINEAGES IN A TEMPERATE BIODIVERSITY HOTSPOT Article Open access 25 June 2024 SUCCESSFUL
POST-GLACIAL COLONIZATION OF EUROPE BY SINGLE LINEAGE OF FRESHWATER AMPHIPOD FROM ITS PANNONIAN PLIO-PLEISTOCENE DIVERSIFICATION HOTSPOT Article Open access 29 October 2020 DISTRIBUTION OF
GENETIC DIVERSITY REVEALS COLONIZATION PATTERNS AND PHILOPATRY OF THE LOGGERHEAD SEA TURTLES ACROSS GEOGRAPHIC SCALES Article Open access 22 October 2020 INTRODUCTION The Pleistocene epoch
(2.5 mya—11 kya) was characterised by extreme climate fluctuations including repeated cold periods with widely extended ice cover and milder periods of glacial retreat1. In temperate areas,
glaciation periods were associated with local population extinctions, southward range shifts (in the northern hemisphere) and resulting genetic bottlenecks2,3. Conversely, milder periods
were associated with rapid northward range expansions2. These historic climatic fluctuations and the associated range shifts left an imprint on the contemporary distribution and genetic
diversity of several taxa in the temperate zone3,4. Recent research also revealed that the current distribution patterns of populations and closely related taxa are associated with species
traits including those related to dispersal5,6. Still, the processes of differentiation and global patterns of gene flow remain largely unexplored in many organisms and ecological groups.
The increasing application of molecular methods and rising number of phylogeographic studies in the last couple of decades facilitated the identification of the most important Pleistocene
refugia across the Palaearctic (e.g. see Hewitt2,7). In Europe, the Balkan, Apennine, and Iberian Peninsulas had the mildest climate during glaciations and acted as important refugia for
many temperate species3,7,8. Consequently, these regions nowadays host high genetic diversity and local endemics8, a recurrent pattern also formulated as the “northern purity vs. southern
richness paradigm”2,9,10. In addition, North Africa11 and the Middle East and its surrounding regions are also identified as possible refugia for several European taxa12,13. Recently, an
increasing number of phylogeographic studies stressed the importance of regions often referred to as ‘cryptic refugia’14,15,16. Cryptic refugia are individual regions located further north
from classical refugia, and are believed to have maintained a suitable and stable environment for the survival of temperate species during glaciations14,17. These regions may have acted as
important hubs from where many species could have expanded their range after the glacial retreat faster than from the southern peninsulas behind geographical barriers, such as mountain
systems and the Mediterranean Sea14. One such extra-Mediterranean example is the Pannonian Plain in Central Europe, which likely acted as a cryptic refugium for several groups, e.g.
crustaceans, amphibians, fish and mammals16,18,19,20,21,22. Each of the Pleistocene refugia could have featured specific environmental conditions and biotic interactions that forced
populations of individual species to adapt locally. Together with the increased importance of drift in isolation23, this has led to genetic differentiation among populations creating the
opportunities for allopatric differentiation processes resulting in intraspecific differentiation and sometimes even speciation2,8. After the glacial retreat, these newly formed evolutionary
units expanded, which led to co-occurrences between sister lineages and species9,24. Several of these genetic lineages are morphologically cryptic, while still representing unique
evolutionary potential (i.e. evolutionary significant unit) and should thus be protected to preserve the adaptive capacity of species25,26,27. The current biodiversity crisis, with its
severe environmental changes and high rates of extinction, calls for a better understanding of this genetic diversity, together with the functioning of habitat networks and genetic
connectivity therein. While terrestrial species could spread relatively fast after the last glacial retreat, quickly filling the new continuous territories opening up in the temperate
regions of Europe7, these post-glacial expansion scenarios may have been fundamentally different for aquatic species inhabiting lentic freshwater ecosystems, such as ponds and lakes. The
discrete nature and patchy distribution of these habitats are major constraints for dispersal in general28. Given that the movements of these taxa in a landscape depend on the availability
of suitable vectors such as wind29,30 or animals5,31,32,33, their re-colonization should be very constrained or stochastic in patchy habitat networks. The so-called “large branchiopods”
(including five extant orders: Anostraca, Notostraca, Spinicaudata, Laevicaudata and Cyclestherida) are well adapted and almost exclusively limited to temporary ponds34,35. They have long
been considered as a flagship group for temporary ponds and their conservation36. Their functional importance, such as community-shaping (anostracans and notostracans37,38,39,40) and
ecosystem engineering (notostracans40) is also increasingly recognized. They produce resting eggs to bridge the unfavourable parts of the year in their local habitats. Their resting eggs can
remain viable buried in the sediment for years before hatching41,42 and play an important role in the passive dispersal of this group. Here, we analyse the genetic diversity of two
congeneric anostracan species (Crustacea, Branchiopoda) belonging to the ancient genus _Branchinecta_, which inhabit temporary ponds across the Palaearctic. We apply a comprehensive approach
that involves sequencing of both mitochondrial and nuclear DNA regions of around 200 specimens from 56 populations (Fig. 1). First, our aim is to determine whether these two species
survived the Pleistocene glaciations in southern refugia, similar to most pond-inhabiting species43,44,45. Second, we want to shed light on the natural history of these closely related
species, and specifically look for signs of allopatric differentiation in separate refugia. Third, we aim to determine post-glacial (re)colonization patterns and discuss the importance of
potential biotic or abiotic vectors that mediated the passive dispersal of the studied anostracan species. The discontinuous (island-like) distribution of _Branchinecta ferox_ and
_Branchinecta orientalis_ populations increases the potential for their genetic isolation and differentiation46. Consequently, we expect to find a rather high genetic variation in both
species on a global scale, especially in geographically isolated regions, while we expect that populations along the main migration routes of water birds (such as North Africa, Iberian
Peninsula and Central Europe) should share common haplotypes. RESULTS GENETIC DIVERSITY Based on the mitochondrial COI DNA region, the mean interspecific divergence between _B. ferox_ and
_B. orientalis_ was 10.1% (Table 1). For _B. ferox_, the overall mean intraspecific genetic variation was 4.0% (Table 1) based on 13 identified haplotypes. One haplotype was relatively
common across Central Europe and was found in 11 populations, while others were mostly present in single populations. The highest genetic divergence (8.4%) was found between a Moroccan
specimen (M1) and another from Israel (IS1). For _B. orientalis_, the overall mean intraspecific genetic variation was 2.9% (Table 1) based on 22 haplotypes. Two haplotypes were relatively
common throughout Central Europe, with one present in 13 and another in 10 populations. Other haplotypes were only recorded from one to three populations each. The highest pairwise distance
(5.8%) was found between one Hungarian specimen (HK4) and individuals from two populations in Austria (AP1, AR4). Based on the nuclear ITS2 DNA region, the mean interspecific distance
between _B. ferox_ and _B. orientalis_ was 11.5% (Table 1). For _B. ferox_, the overall mean intraspecific genetic differentiation was 3.7% (Table 1) considering 3 haplotypes. One haplotype
was widely distributed across Central Europe and present in Spain and Morocco. For _B. orientalis_, the overall mean intraspecific genetic differentiation was 1.5% (Table 1) considering 7
haplotypes. Three (out of four) haplotypes in Central Europe were rare and each was present in only one population. For the nuclear 18S region, we generated 16 sequences (five sequences of
_B. ferox_ and 11 sequences of _B. orientalis_) of 598 to 1719 bp. We added two further _B. orientalis_ sequences from China to the set of sequences47. We found only one mutation (C or T)
between the two examined _Branchinecta_ species on the nuclear 18S region. No variability was observed within _B. ferox_. In _B. orientalis_, we found genetic variation at only two bases
between the Mongolian and all other specimens. The existence of one more haplotype identified by Deng et al.47 is hence possibly a misinterpretation that occurred via a mistake in generating
the sequence MW829399, as there is a T nucleotide insertion close to the end of the sequence. PHYLOGENETIC ANALYSES At mitochondrial COI DNA region, both methods of phylogenetic inference
(maximum likelihood [ML] and Bayesian inference [BI]) produced trees with similar topologies. _B. ferox_ (Fig. 2a, b) populations can be subdivided into five main haplogroups, corresponding
to distinct geographical regions. These haplogroups represent the Middle East, two regions in Northern Africa (Tunisia vs. Morocco) and two in Europe (Spain vs. Spain and Central Europe).
One group in Europe included specimens from the Albacete region in south-east Spain and the second included populations from Segovia in central Spain and all populations from Central Europe.
For _B. orientalis_ (Fig. 2a, c), the phylogenetic search methods (ML and BI) grouped the studied _B. orientalis_ haplotypes in two larger haplogroups (Clade A & B). Populations from
Central Europe were present in both haplogroups. Most of the Spanish individuals (except for one specimen from Cuenca region, east-central Spain) belonged to Clade B. Both phylogenetic
reconstructions of nuclear ITS2 DNA region first differentiated _B. ferox_ from _B. orientalis_ species (Fig. A1 in Appendix C). Within _B. ferox_, it was possible to separate three groups,
one in the Middle East (Israel), another in the Iberian Peninsula (Albacete), and a third from North Africa (Morocco), the Iberian Peninsula (Segovia) and Central Europe. Unfortunately, no
ITS2 sequences were obtained from the Tunisian population of the species. For _B. orientalis_, reconstructions based on the ITS2 region suggested slightly different phylogenetic
relationships compared to the COI DNA sequences. The sequences from Spain and both Mongolia and China were grouped together but were further subdivided into two distinctive haplogroups
matching the geographic origin of the samples. Except for the two sequences originating from two populations in Austria and Serbia (AW1 and SO4) that formed their own haplogroup, all
populations from Central Europe belonged to another haplogroup. GENETIC AND SPATIAL DISTANCE BASED ON THE MITOCHONDRIAL COI GENE REGION A significant distance-decay relationship was revealed
for pairwise genetic distances (K2P) of both _B. orientalis_ (Fig. 3a; Mantel test: r = 0.22, _p_ = 0.001) and _B. ferox_ (Fig. 3b; Mantel test: r = 0.65, _p_ = 0.001). This was also
significant in the two clades (Clades A and B of _B. orientalis_) when tested separately (Fig. 3a). In Clade A, which included populations from the Russian Federation, China, and Mongolia,
we found a stronger relationship with spatial distance (r = 0.53, _p_ = 0.001) than in the entire European Clade B (r = 0.26, _p_ = 0.001). In agreement with the steeper slope of
distance-decay in _B. ferox_, the signs of autocorrelation were also stronger in this species based on Mantel correlations. Here, we found positive autocorrelation between genetic distances
and spatial distances within 100 km (Fig. 4b), which turned negative over larger spatial scales. A similar trend was found in _B. orientalis_, with most positive relationships within 100 km
(Fig. 4a), but in the entire dataset as well as in Clade A, we found two cases of significant positive correlation even on the larger scale (> 100 km), indicating successful long-distance
dispersal events. DISCUSSION Our study illustrates how historical long-term isolation can have a lasting impact on the current distribution and genetic diversity of sibling species. Even
though the present distribution of the congeneric anostracans _Branchinecta ferox_ and _B. orientalis_ overlap substantially within Europe, our results suggest that this was not the case
during the Pleistocene. Moreover, our data on current regional genetic diversities provided evidence for the existence of multiple Pleistocene refugial regions for both species. This has
resulted in distinct and well-characterised evolutionary lineages within the two species, both through new mutations and lineage sorting acting on their pre-existing genetic variability,
revealing a history of allopatric differentiation. Based on haplotypes shared between geographically distant regions, we could detect several recent long-distance dispersal events (> 1000
km). At the same time, our results based on population similarities implied that most dispersal events predominantly happen on a much smaller spatial scale (within 100 km). This is in good
agreement with the growing evidence that the realized dispersal in passively dispersing large branchiopods is routinely occurring at the scale of few tens of km, with higher values in arid
areas and open grasslands, and lower values for the species occurring in forested areas (e.g.48,49,50). In our study, we applied multiple molecular markers to unravel the species history of
the studied sibling species of _Branchinecta_: one mitochondrial (COI) and two nuclear (ITS2 and 18S). They generally point to the allopatric speciation of the two species, as well as to the
reason behind their intraspecific differentiation: their survival in different Palearctic refugia during the Pleistocene. Applying the evolutionary rates for COI51, the most recent common
ancestor of the two species is to be located at around 5.69 mya, i.e. in the late Miocene or in the Pliocene. In _B. ferox_ populations, differentiation likely started earlier than in _B.
orientalis_, as the North African and the Middle East clades themselves are well differentiated from each other (around 8% distance on COI region and 5% on ITS2 region), and the major clades
were separable based on both the COI and ITS2 DNA regions. All COI haplotypes in _B. orientalis_ were classified into two haplogroups, while in the ITS2 tree, divergence was generally low.
The slightly different tree topologies found for these two markers (especially in Central Europe, that holds a large number of local populations of _B. orientalis_) could be explained by a
relatively recent split between the two clades combined with the different inheritance mechanisms: while mitochondrial genes are inherited from the mothers (but see exceptions to that rule
in Lindholm et al.51), nuclear genes are typically inherited from both parents. As we found generally higher phylogeographic diversity (both based on COI and ITS2 DNA) of _B. ferox_ in the
southern parts of its current distribution range, this indicated multiple potential refugia in this region (i.e., Northern Africa, the Middle East, and possibly Spain). The Mediterranean
areas of Africa and Asia were not covered by ice during the Pleistocene and provided suitable habitats to serve as refugia for many species8,11,24 including temporary pond-dwellers, e.g.,
copepods10 and other anostracan species43. From the southern Mediterranean region, _B. ferox_ possibly (re)colonized the Iberian Peninsula first as supported by the presence of two
haplogroups for both genetic markers (Fig. 2a & Fig. A1). Moreover, the (re)colonization of the Iberian Peninsula (i.e., Europe) might have started long before the last glacial maximum.
Another possibility is that _B. ferox_ was present in the Iberian Peninsula throughout the Pleistocene in which case the Iberian Peninsula would have acted as another Pleistocene refugium
for _B. ferox_. For _B. orientalis_, our phylogeographic data suggest a Pleistocene refugium located in the Pannonian Plain or in a nearby region, e.g., the Balkan Peninsula, which is a much
more common refugium for several species; however, given that _B. orientalis_ currently only occurs in the Pannonian Plain, we suggest this as the more likely scenario. This hypothesis is
supported by the relatively high diversity observed on both genetic markers in Central Europe and by the topology of the constructed haplotype network. The presence of two
well-differentiated COI clades on the Pannonian Plain indicates the existence of two separated populations—possibly due to a “refugia within refugium” pattern24—during the glaciation
periods. Based on the COI gene region, we can assume that all current _B. orientalis_ populations originated from the Pannonian Plain (and/or the Balkan Peninsula); it implies that
colonization of the Iberian Peninsula happened through at least two distinct events of colonization. This is suggested by the fact that on the Iberian Peninsula all samples except for one
specimen belonged to one of two identified COI haplogroups. In this case, the re-colonization route we revealed from the Pannonian Plain to the Iberian Peninsula is quite unique for
Palaearctic species in general: all other taxa that had a refugium in the Pannonian Plain during the Pleistocene have a much more restricted range today (e.g. amphibians21,52). It is also
possible that the colonization of the Iberian Peninsula and Central Asia happened during one of the previous interglacial periods (as also suggested for cladocerans53). In particular, most
populations in Central Asia constitute a separate (sub)clade based on the sequenced COI and ITS2 regions and some even show minor differences on the 18S region in comparison to the other
populations of _B. orientalis_, which supports this possibility. Our genetic data showed strong bottleneck effects of the Pleistocene glaciations in both species. In _B. ferox_, there was a
very clear spatial structure visible on the phylogenetic tree, and it was even more explicitly shown in the spatial analyses in _B. orientalis_ and _B. ferox_ (based on both distance decay
and spatial autocorrelation analyses). Aquatic passive dispersers often show clear geographic structuring (e.g. in _Branchipus schaefferi_43 and _Triops cancriformis_54), which is also the
case with the two studied _Branchinecta_ species (see Figs. 3 and 4). At the same time, there can be marked differences in the prevalence of long-distance dispersal events between the
species with distinct habitat requirements. For species occupying smaller ephemeral habitats, wind can be a more important dispersal vector55, with dispersal events mostly happening within
very short distances29,30,56,57. Such species include most anostracans (e.g., _Branchipodopsis wolfi_56 and notostracans (_Triops cancriformis_54), where indeed there is no or very scarce
indication for long-distance dispersal events. The upper end of this “mobility” gradient is the anostracan genus _Artemia_ frequently inhabiting larger waterbodies: here long-distance
dispersal by waterbirds feeding on _Artemia_58 (and even by humans59) leads to gene flow detectable even at larger scales60,61. Our study species are somewhere between these two extremes,
with visible geographic structuring but with several possible long-distance dispersal events visible in the data (e.g., several extremely low values of genetic distance over 1000 km in _B.
orientalis_ or the Spanish _B. ferox_ population clustering together with populations from the Pannonian Plain). Here, we found that most dispersal events in both _Branchinecta_ species have
been happening within 100 km. This distance is well in accordance with the dispersal habits of most waterbirds62,63. The role of waterbirds as dispersal agents is well documented for many
aquatic invertebrates, including anostracans31,62,64,65,66. The studied _Branchinecta_ species in Central Europe and Spain inhabit shallow sodic lakes of a relatively large surface
area67,68, situated along the seasonal migration routes of a diverse set of waterbird species69, among which several are even proven to be attracted to habitats with the most abundant
_Branchinecta_ populations70. This altogether implies the dominant role of waterbirds connecting local populations. Right after the glacial retreat, the dispersal of _Branchinecta_ could
have been further facilitated by historic long-distance mammal migrations, specifically related to the extinct megafauna71, e.g. mammoth species (_Mammuthus_ spp.), that are known to carry
diverse propagules including _Branchinecta_ resting eggs on their body72. They once inhabited a vast area of Eurasia and moved over large distances73,74, similar to their extant sibling
species the African elephants, also known as vectors for passive dispersers75. However, nowadays large mammals can only contribute to small-scale dispersal events33,76. Finally, the high
genetic diversity of _B. orientalis_ in eastern Austria underlines the importance of dense habitat networks for maintaining both local and regional genetic diversity, which is further
supported by our results showing high dispersal rates within smaller regions (< 100 km). This calls for adequate protection of the inhabitants of temporary aquatic systems on the regional
(metapopulation) level, together with their aquatic habitats. Moreover, particular attention should be paid to the long-term conservation of the patchily distributed and isolated
populations of _B. ferox_ in Africa and Asia, which host unique and well-differentiated endemic lineages currently at risk of extinction. The realisation of conservation programmes aimed at
the long-term persistence of these significant evolutionary units is strongly desirable. METHODS STUDY SPECIES The anostracan genus _Branchinecta_ comprises around 50 species. Its members
are found on all continents except Australia77,78,79. The genus is represented by five species in the Palaearctic, with only two being present in the study area: _Branchinecta ferox_ and
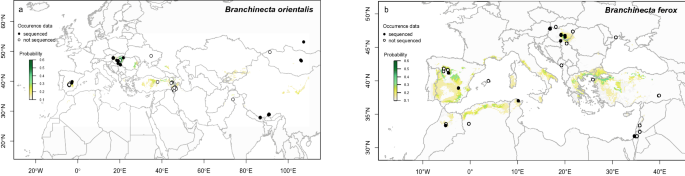
_Branchinecta orientalis_. _B. orientalis_ inhabits mineral-rich temporary waters and has a disjunct distribution ranging between 27° and 55° N in Europe and Asia (Fig. 1a). Active
populations generally occur in spring, but they have also been recorded in autumn or winter80,81,82. _B. ferox_ has a circum-Mediterranean and Central European distribution (Fig. 1b). It is
the only _Branchinecta_ species occurring in Africa, being present in the north-western part of the continent (Morocco, Algeria and Tunisia78). In Europe, it occurs in Spain and Central
Europe (Pannonian Plain), and its range extends further east across South Ukraine to the west of Russia83,84. This species has also been reported in the Middle-East (i.e. Jordan, Israel and
Turkey85,86,87,88). _B. ferox_ is a halotolerant species, occurring both in freshwater rain pools in the circum-Mediterranean area85 and saline pans in the Pannonian Plain68. Active
populations mostly occur in late winter and early spring68,78,81. The geographic distribution of these two _Branchinecta_ species overlaps in the Pannonian Plain, Iberian Peninsula and
Turkey68,87,89. On the Iberian Peninsula and the Pannonian Plain, the two species are found almost exclusively in large and shallow saline pans, and represent a preferred food source for
waterbirds on their seasonal migration routes70. SPECIES DISTRIBUTION MAPS We compiled a list of known occurrences of both species based on the above listed samples and literature
data47,67,68,78,83,84,85,86,87,89,90,91,92,93,94,95,96,97,98,99,100. The literature sources mentioning distribution and ecology of _B. ferox_ and _B. orientalis_ were searched via Google
Scholar and Web of Science. Sources that did not report precise habitat coordinates of populations and/or are older than 50 years are not included, hence the actual distribution of the
species is probably underrepresented (e.g., the actual distribution of _B. orientalis_ in Asia is most likely underrepresented here). To account for this, we built species distribution maps
with the ‘dismo’ package101 of R v. 4.0.3102. Here, we used all available bioclimatic variables from the WorldClim database (http://www.worldclim.org)103, and predicted the probability of
occurrence for each species. Although these variables do not include the presence of suitable habitats (i.e., shallow temporary waters, for which there is no publicly available database
yet), they should provide a reliable indication for the climatic conditions where suitable habitats are likely to occur. According to the probability maps, our general coverage of sequenced
samples was in a good agreement with the overall distribution of both species, including samples from the Mediterranean, the Pannonian Plain in Central Europe (both species), and Middle to
Central Asia (_B. orientalis_). Even though our model predicted the possible occurrence of _B. ferox_ in Italy and Southern France (Fig. 1b), we can mostly exclude these latter regions given
that both are very well covered by previous Anostraca studies that have never reported the species there104,105. SAMPLING PROCEDURE We collected _Branchinecta orientalis_ specimens from 29
temporary pools, ponds and shallow lakes in Europe and Asia (Table A1). _Branchinecta ferox_ specimens were collected from 16 habitats in Europe, North Africa, and Asia (Table A1). Specimens
were collected between 1971 and 2018 and fixed in ethanol (of various concentrations). Once the samples arrived at the lab, animals were transferred immediately to pure ethanol until
further processing. All specimens were dissected to obtain phyllopod tissue for DNA extraction. For the molecular laboratory procedures to acquire the DNA sequences for the targeted gene
regions, see Appendix B. RECONSTRUCTIONS OF PHYLOGENY BASED ON MITOCHONDRIAL COI AND NUCLEAR ITS2 DNA REGION All generated _B. ferox and B. orientalis_ sequences were assembled and visually
checked for quality in SeqScape v3. We checked the COI alignment for indels and internal stop codons that would indicate unintentional amplification of nuclear pseudogenes106. The produced
sequences were edited in BioEdit107. The newly produced sequences were aligned together with the existing sequences in GenBank (for _B. ferox_ and _B. orientalis_ see Table A1 in Appendix
1A; _Branchinecta lynchi_ MF037649; _B. lindahli_ MF037694-5; _B. tolli_ HG797695; _B. paludosa_ HG797672, HG797699 and JN233828)47,51,89,96,108,109 and one outgroup taxon (for COI, we used
_Branchipus schaefferi_ MK44941643 and for ITS2, _Chirocephalus diaphanus_ LT86020689) by using CLUSTALW multiple alignment tool in BioEdit for the CO1 gene region and MUSCLE for the ITS2
DNA region. The most likely evolutionary model for the COI marker was determined in in PartitionFinder2110 and for the ITS2 in MEGA X111 based on the Akaike Information Criterion (AIC). For
the COI gene region, the AIC selected a General Time Reversible model (GTR), which was used to reconstruct ML and BI tree. For the ITS2 DNA region, the AIC selected for GTR model with a
gamma shape parameter (+ G, γ = 1.22), which was used to reconstruct ML and BI tree. ML analyses were performed in MEGA X with 1000 bootstrap replicates. Bayesian inference was performed in
BEAST v2.6.4112 in case of the COI gene region. The settings included the strict molecular clock, Yule model and a lognormal prior distribution for the taxon set of the _Branchinecta
paludosa_ samples (set as monophyletic; mean ± standard deviation: 1.25 ± 0.15 as in Lindholm et al.51). The analyses were run for 10 million generations. Molecular evolutionary rates of 2%
divergence per million years were applied by Lindholm et al.51 on the closely related _B. paludosa_, and were thus here applied to get a tentative temporal frame for the main cladogenetic
events observed within our study taxa. We used TreeAnnotator v. 2.6.4 to construct a single tree by discarding 25% of the compiled trees as a burn-in. As molecular clock is not available for
the ITS2 DNA region, we used MrBayes113,114,115 to an ITS2 phylogenetic tree using BI. We applied the Markov Chain Monte Carlo (MCMC) method for 106 generations (standard deviation of split
frequencies reached < 0.01) while the trees were sampled every 1000 generations. The initial 25% of produced trees were discarded as burn-in. For the _B. ferox_ and _B. orientalis_ COI
gene fragments, we built a median-joining haplotype network for each species (ε = 0; Bandelt et al., 1999) using PopART v 1.7117; http://popart.otago.ac.nz). The sites containing missing
bases at the end and the beginning of the alignment, as well as ambiguous bases, were masked leaving 479 (_B. ferox_) and 304 (_B. orientalis_) sites for further network analysis. ANALYSIS
OF GENETIC DIVERSITY Substitution saturation was tested in DAMBE v. 7.0.28118, using the default settings and including all sites. The index of substitution saturation (Iss) was
significantly smaller than the critical index of substitution saturation (Iss c), indicating little saturation119,120 for both markers. Pairwise genetic K2P distances between all generated
sequences and the mean genetic distances within and among the main groups in the phylogeny of _B. ferox and B. orientalis_ were calculated in MEGA X121 with partial deletion of 90% (515
positions in the final data set for COI and 574 positions for ITS2). The haplotype number was determined in DnaSP 6122. In both _B. ferox_ and _B. orientalis_, we tested for the dispersal
limitation based on the relationship between pairwise genetic differences on the mitochondrial COI gene region and geographic distances. To do so, we exported pairwise genetic distances from
MEGA X in a form of a data matrix and applied Hellinger transformation. We calculated pairwise geographic distances between all sampling sites as orthodromic distance. To reveal effective
dispersal over distinct distance classes, we used the computed pairwise genetic distances and log + 0.1 transformed spatial distances to perform a Mantel test with 999 permutations and
calculate Mantel correlation coefficients. In addition to the full dataset, separate Mantel tests were performed within two main _B. orientalis_ clades (Clade A and Clade B). Mantel
correlation coefficients were calculated between pairwise genetic distances within eight distance classes for all COI sequences of _B. orientalis_ and repeated separately for the two main
clades to detect positive autocorrelation as signs of effective dispersal. For _B. ferox_, we calculated Mantel correlation coefficients between pairwise genetic distances within seven
distance classes as the highest spatial distance between _B. ferox_ populations was lower than between individual _B. orientalis_ populations. Calculation of pairwise spatial distances,
Mantel tests and Mantel correlation coefficients were performed in R software, with the ‘fields’123 and ‘vegan’124 packages. DATA ACCESSIBILITY The DNA sequence data supporting the findings
of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/, accession numbers are listed in the Appendix A, Table A1. REFERENCES * Paillard, D. The timing of
Pleistocene glaciations from a simple multiple-state climate model. _Nature_ 391, 378–381 (1998). ADS Google Scholar * Hewitt, G. M. Genetic consequences of climatic oscillations in the
Quaternary. _Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci._ 359, 183–195 (2004). CAS Google Scholar * Hewitt, G. The genetic legacy of the quaternary ice ages. _Nature_ 405, 907–913
(2000). ADS CAS PubMed Google Scholar * Taberlet, P., Fumagalli, L., Wust-Saucy, A.-G. & Cosson, J.-F. Comparative phylogeography and postglacial colonization routes in Europe. _Mol.
Ecol._ 7, 453–464 (1998). CAS PubMed Google Scholar * Incagnone, G., Marrone, F., Barone, R., Robba, L. & Naselli-Flores, L. How do freshwater organisms cross the ‘dry ocean’? A
review on passive dispersal and colonization processes with a special focus on temporary ponds. _Hydrobiologia_ 750, 103–123 (2015). Google Scholar * Schmitt, T. & Varga, Z.
Extra-Mediterranean refugia: The rule and not the exception?. _Front Zool_ 9, 22 (2012). PubMed PubMed Central Google Scholar * Hewitt, G. M. Speciation, hybrid zones and
phylogeography—Or seeing genes in space and time. _Mol. Ecol._ 10, 537–549 (2001). CAS PubMed Google Scholar * Habel, J. C., Drees, C., Schmitt, T. & Assmann, T. Review refugial areas
and postglacial colonizations in the Western Palearctic. In _Relict Species_ (eds Habel, J. C. & Assmann, T.) 189–197 (Springer, 2010). Google Scholar * Hewitt, G. Some genetic
consequences of ice ages, and their role in divergence and speciation. _Biol. J. Lin. Soc._ 58, 247–276 (1996). Google Scholar * Marrone, F., Lo Brutto, S. & Arculeo, M. Molecular
evidence for the presence of cryptic evolutionary lineages in the freshwater copepod genus _Hemidiaptomus_ G.O. Sars, 1903 (Calanoida, Diaptomidae). _Hydrobiologia_ 644, 115–125 (2010). CAS
Google Scholar * Husemann, M., Schmitt, T., Zachos, F. E., Ulrich, W. & Habel, J. C. Palaearctic biogeography revisited: Evidence for the existence of a North African refugium for
Western Palaearctic biota. _J. Biogeogr._ 41, 81–94 (2014). Google Scholar * García-Vázquez, D., Bilton, D. T., Foster, G. N. & Ribera, I. Pleistocene range shifts, refugia and the
origin of widespread species in western Palaearctic water beetles. _Mol. Phylogenet. Evol._ 114, 122–136 (2017). PubMed Google Scholar * Perktas, U., Barrowclough, G. F. & Groth, J. G.
Phylogeography and species limits in the green woodpecker complex (Aves: Picidae): Multiple Pleistocene refugia and range expansion across Europe and the Near East. _Biol. J. Lin. Soc._
104, 710–723 (2011). Google Scholar * Stewart, J. R. & Lister, A. M. Cryptic northern refugia and the origins of the modern biota. _Trends Ecol. Evol._ 16, 608–613 (2001). Google
Scholar * Stewart, J. R., Lister, A. M., Barnes, I. & Dalén, L. Refugia revisited: Individualistic responses of species in space and time. _Proc. R. Soc. B Biol. Sci._ 277, 661–671
(2010). Google Scholar * Sworobowicz, L., Mamos, T., Grabowski, M. & Wysocka, A. Lasting through the ice age: The role of the proglacial refugia in the maintenance of genetic diversity,
population growth, and high dispersal rate in a widespread freshwater crustacean. _Freshw. Biol._ 65, 1028–1046 (2020). CAS Google Scholar * Provan, J. & Bennett, K. D.
Phylogeographic insights into cryptic glacial refugia. _Trends Ecol. Evol._ 23, 564–571 (2008). PubMed Google Scholar * Antal, L. _et al._ Phylogenetic evidence for a new species of
_Barbus_ in the Danube River basin. _Mol. Phylogenet. Evol._ 96, 187–194 (2016). CAS PubMed Google Scholar * Copilaş-Ciocianu, D., Fišer, C., Borza, P. & Petrusek, A. Is subterranean
lifestyle reversible? Independent and recent large-scale dispersal into surface waters by two species of the groundwater amphipod genus _Niphargus_. _Mol. Phylogenet. Evol._ 119, 37–49
(2018). PubMed Google Scholar * Říčanová, Š _et al._ Multilocus phylogeography of the European ground squirrel: Cryptic interglacial refugia of continental climate in Europe. _Mol. Ecol._
22, 4256–4269 (2013). PubMed Google Scholar * Vörös, J., Mikulíček, P., Major, Á., Recuero, E. & Arntzen, J. W. Phylogeographic analysis reveals northerly refugia for the riverine
amphibian _Triturus dobrogicus_ (Caudata: Salamandridae). _Biol. J. Linn. Soc._ 119, 974–991 (2016). Google Scholar * Wielstra, B. _et al._ Tracing glacial refugia of Triturus newts based
on mitochondrial DNA phylogeography and species distribution modeling. _Front. Zool._ 10, 13 (2013). PubMed PubMed Central Google Scholar * Hutchison, D. W. & Templeton, A. R.
Correlation of pairwise genetic and geographic distance measures: Inferring the relative influences of gene flow and drift on the distribution of genetic variability. _Evolution_ 53,
1898–1914 (1999). PubMed Google Scholar * Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. _Front. Zool._ 4, 11 (2007). PubMed PubMed Central
Google Scholar * Ewart, K. M. _et al._ Phylogeography of the iconic Australian red-tailed black-cockatoo (_Calyptorhynchus banksii_) and implications for its conservation. _Heredity_ 125,
85–100 (2020). PubMed PubMed Central Google Scholar * Hutama, A. _et al._ Identifying spatially concordant evolutionary significant units across multiple species through DNA barcodes:
Application to the conservation genetics of the freshwater fishes of Java and Bali. _Glob. Ecol. Conserv._ 12, 170–187 (2017). Google Scholar * Médail, F. & Baumel, A. Using
phylogeography to define conservation priorities: The case of narrow endemic plants in the Mediterranean Basin hotspot. _Biol. Cons._ 224, 258–266 (2018). Google Scholar * Previšić, A.,
Walton, C., Kučinić, M., Mitrikeski, P. T. & Kerovec, M. Pleistocene divergence of Dinaric _Drusus_ endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan
Peninsula. _Mol. Ecol._ 18, 634–647 (2009). PubMed Google Scholar * Brendonck, L. & Riddoch, B. J. Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). _Biol.
J. Linn. Soc._ 67, 87–95 (1999). Google Scholar * Horváth, Z., Vad, C. F. & Ptacnik, R. Wind dispersal results in a gradient of dispersal limitation and environmental match among
discrete aquatic habitats. _Ecography_ 39, 726–732 (2016). PubMed Google Scholar * Brochet, A. L. _et al._ Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal
(_Anas crecca_) in the Camargue (southern France). _Hydrobiologia_ 637, 255 (2009). Google Scholar * Figuerola, J. & Green, A. J. Dispersal of aquatic organisms by waterbirds: A review
of past research and priorities for future studies. _Freshw. Biol._ 47, 483–494 (2002). Google Scholar * Vanschoenwinkel, B. _et al._ Dispersal of freshwater invertebrates by large
terrestrial mammals: A case study with wild boar (_Sus scrofa_) in Mediterranean wetlands. _Freshw. Biol._ 53, 2264–2273 (2008). Google Scholar * Brendonck, L., Rogers, D. C., Olesen, J.,
Weeks, S. & Hoeh, W. R. Global diversity of large branchiopods (Crustacea : Branchiopoda) in freshwater. _Hydrobiologia_ 595, 167–176 (2008). Google Scholar * Dumont, H. J. &
Negrea, S. V. _Introduction to the Class Branchiopoda_. (Backhuys Publishers, 2002). * Belk, D. Global status and trends in ephemeral pool invertebrate conservation: Implications for
Californian fairy shrimp. In _Ecology, Conservation, and Management of Vernal Pool Ecosystems—Proceedings from a 1996 conference_ 147–150 (California Native Plant Society, 1998). * Jocque,
M., Vanschoenwinkel, B. & Brendonck, L. Anostracan monopolisation of early successional phases in temporary waters?. _Fundam. Appl. Limnol._ 176, 127–132 (2010). Google Scholar * Lukić,
D., Horváth, Z., Vad, C. F. & Ptacnik, R. Food spectrum of _Branchinecta orientalis_—Are anostracans omnivorous top consumers of plankton in temporary waters?. _J. Plankton Res._ 40,
436–445 (2018). Google Scholar * Lukić, D., Ptacnik, R., Vad, C. F., Pόda, C. & Horváth, Z. Environmental constraint of intraguild predation: Inorganic turbidity modulates omnivory in
fairy shrimps. _Freshw. Biol._ 65, 226–239 (2020). Google Scholar * Waterkeyn, A., Grillas, P., Anton-Pardo, M., Vanschoenwinkel, B. & Brendonck, L. Can large branchiopods shape
microcrustacean communities in Mediterranean temporary wetlands?. _Mar. Freshw. Res._ 62, 46–53 (2011). CAS Google Scholar * Brendonck, L. & De Meester, L. Egg banks in freshwater
zooplankton: Evolutionary and ecological archives in the sediment. _Hydrobiologia_ 491, 65–84 (2003). Google Scholar * Hairston, N. G., Brunt, R. A. V., Kearns, C. M. & Engstrom, D. R.
Age and survivorship of diapausing eggs in a sediment egg bank. _Ecology_ 76, 1706–1711 (1995). Google Scholar * Lukić, D. _et al._ High genetic variation and phylogeographic relations
among Palearctic fairy shrimp populations reflect persistence in multiple southern refugia during Pleistocene ice ages and postglacial colonisation. _Freshw. Biol._ 64, 1896–1907 (2019).
Google Scholar * Marrone, F., Alfonso, G., Naselli-Flores, L. & Stoch, F. Diversity patterns and biogeography of Diaptomidae (Copepoda, Calanoida) in the Western Palearctic.
_Hydrobiologia_ 800, 45–60 (2017). CAS Google Scholar * Vanschoenwinkel, B. _et al._ Toward a global phylogeny of the "living fossil’’ crustacean order of the Notostraca. _PLos ONE_
7, e34998 (2012). * Boileau, M. & Hebert, P. Genetic consequences of passive dispersal in pond-dwelling Copepods. _Evolution_ 45, 721–733 (1991). PubMed Google Scholar * Deng, Z.,
Chen, Y., Ma, X., Hu, W. & Yin, M. Dancing on the top: Phylogeography and genetic diversity of high-altitude freshwater fairy shrimps (Branchiopoda, Anostraca) with a focus on the
Tibetan Plateau. _Hydrobiologia_ 848, 2611–2626 (2021). CAS Google Scholar * Ketmaier, V. _et al._ Mitochondrial DNA regionalism and historical demography in the extant populations of
_Chirocephalus kerkyrensis_ (Branchiopoda: Anostraca). _PLoS ONE_ 7, e30082 (2012). ADS CAS PubMed PubMed Central Google Scholar * Korn, M. _et al._ Phylogeny, molecular ecology and
taxonomy of southern Iberian lineages of _Triops mauritanicus_ (Crustacea: Notostraca). _Org. Divers. Evol._ 10, 409–440 (2010). Google Scholar * Stoch, F., Korn, M., Turki, S.,
Naselli-Flores, L. & Marrone, F. The role of spatial environmental factors as determinants of large branchiopod distribution in Tunisian temporary ponds. _Hydrobiologia_ 782, 37–51
(2016). Google Scholar * Lindholm, M., d’Auriac, M. A., Thaulow, J. & Hobaek, A. Dancing around the pole: Holarctic phylogeography of the Arctic fairy shrimp _Branchinecta paludosa_
(Anostraca, Branchiopoda). _Hydrobiologia_ 772, 189–205 (2016). CAS Google Scholar * Vörös, J., Alcobendas, M., Martínez-Solano, I. & García-París, M. Evolution of _Bombina bombina_
and _Bombina variegata_ (Anura: Discoglossidae) in the Carpathian Basin: A history of repeated mt-DNA introgression across species. _Mol. Phylogenet. Evol._ 38, 705–718 (2006). PubMed
Google Scholar * Zharov, A. A. _et al._ Pleistocene branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia demonstrate morphological and ecological stasis. _Water_ 12, 3063 (2020).
Google Scholar * Velonà, A., Luchetti, A., Scanabissi, F. & Mantovani, B. Genetic variability and reproductive modalities in European populations of _Triops cancriformis_ (Crustacea,
Branchiopoda, Notostraca). _Ital. J. Zool._ 76, 366–375 (2009). Google Scholar * Vanschoenwinkel, B., Gielen, S., Vandewaerde, H., Seaman, M. & Brendonck, L. Relative importance of
different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. _Ecography_ 31, 567–577 (2008). Google Scholar * Hulsmans, A., Moreau, K., Meester, L. D., Riddoch,
B. J. & Brendonck, L. Direct and indirect measures of dispersal in the fairy shrimp _Branchipodopsis wolfi_ indicate a small scale isolation-by-distance pattern. _Limnol. Oceanogr._ 52,
676–684 (2007). ADS Google Scholar * Vanschoenwinkel, B., Vries, C. D., Seaman, M. & Brendonck, L. The role of metacommunity processes in shaping invertebrate rock pool communities
along a dispersal gradient. _Oikos_ 116, 1255–1266 (2007). Google Scholar * Sánchez, M. I., Green, A. J., Amat, F. & Castellanos, E. M. Transport of brine shrimps via the digestive
system of migratory waders: Dispersal probabilities depend on diet and season. _Mar. Biol._ 151, 1407–1415 (2007). Google Scholar * Horváth, Z. _et al._ Eastern spread of the invasive
_Artemia franciscana_ in the Mediterranean Basin, with the first record from the Balkan Peninsula. _Hydrobiologia_ 822, 229–235 (2018). Google Scholar * Muñoz, J., Amat, F., Green, A. J.,
Figuerola, J. & Gómez, A. Bird migratory flyways influence the phylogeography of the invasive brine shrimp _Artemia franciscana_ in its native American range. _PeerJ_ 1, e200 (2013).
PubMed PubMed Central Google Scholar * Muñoz, J. _et al._ Phylogeography and local endemism of the native Mediterranean brine shrimp _Artemia salina_ (Branchiopoda: Anostraca). _Mol.
Ecol._ 17, 3160–3177 (2008). PubMed Google Scholar * Sánchez, M. I., Hortas, F., Figuerola, J. & Green, A. J. Comparing the potential for dispersal via waterbirds of a native and an
invasive brine shrimp. _Freshw. Biol._ 57, 1896–1903 (2012). Google Scholar * Viana, D. S., Santamaría, L., Michot, T. C. & Figuerola, J. Migratory strategies of waterbirds shape the
continental-scale dispersal of aquatic organisms. _Ecography_ 36, 430–438 (2013). Google Scholar * Green, A. J. _et al._ Dispersal of invasive and native brine shrimps _Artemia_ (Anostraca)
via waterbirds. _Limnol. Oceanogr._ 50, 737–742 (2005). ADS Google Scholar * Kappas, I. _et al._ Molecular and morphological data suggest weak phylogeographic structure in the fairy
shrimp _Streptocephalus torvicornis_ (Branchiopoda, Anostraca). _Hydrobiologia_ 801, 21–32 (2017). CAS Google Scholar * Rogers, D. C. Larger hatching fractions in avian dispersed
anostracan eggs (Branchiopoda). _J. Crustac. Biol._ 34, 135–143 (2014). Google Scholar * Angeler, D. G., Viedma, O., Sánchez-Carrillo, S. & Alvarez-Cobelas, M. Conservation issues of
temporary wetland Branchiopoda (Anostraca, Notostraca: Crustacea) in a semiarid agricultural landscape: What spatial scales are relevant?. _Biol. Cons._ 141, 1224–1234 (2008). Google Scholar
* Horváth, Z., Vad, C. F., Vörös, L. & Boros, E. Distribution and conservation status of fairy shrimps (Crustacea: Anostraca) in the astatic soda pans of the Carpathian basin: the role
of local and spatial factors. _J. Limnol._ 72, 103–116 (2013). Google Scholar * Svensson, L., Mullarney, K. & Zetterström, D. _Collins Bird Guide_ 2nd edn. (HarperCollins Publishers
Ltd., 2009). Google Scholar * Horváth, Z., Vad, C. F., Vörös, L. & Boros, E. The keystone role of anostracans and copepods in European soda pans during the spring migration of
waterbirds. _Freshw. Biol._ 58, 430–440 (2013). Google Scholar * Gill, J. L. Ecological impacts of the late Quaternary megaherbivore extinctions. _New Phytol._ 201, 1163–1169 (2014). PubMed
Google Scholar * Neretina, A. N. _et al._ Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the
Pleistocene. _Sci. Rep._ 10, 859 (2020). ADS CAS PubMed PubMed Central Google Scholar * Chang, D. _et al._ The evolutionary and phylogeographic history of woolly mammoths: A
comprehensive mitogenomic analysis. _Sci. Rep._ 7, 44585 (2017). ADS CAS PubMed PubMed Central Google Scholar * Lister, A. M., Sher, A. V., van Essen, H. & Wei, G. The pattern and
process of mammoth evolution in Eurasia. _Quatern. Int._ 126–128, 49–64 (2005). Google Scholar * Vanschoenwinkel, B. _et al._ Passive external transport of freshwater invertebrates by
elephant and other mud-wallowing mammals in an African savannah habitat. _Freshw. Biol._ 56, 1606–1619 (2011). Google Scholar * Waterkeyn, A., Pineau, O., Grillas, P. & Brendonck, L.
Invertebrate dispersal by aquatic mammals: A case study with nutria _Myocastor coypus_ (Rodentia, Mammalia) in Southern France. _Hydrobiologia_ 654, 267–271 (2010). Google Scholar * Belk,
D. & Brtek, J. Checklist of the Anostraca. _Hydrobiologia_ 298, 315–353 (1995). Google Scholar * Marrone, F., Korn, M., Stoch, F., Naselli Flores, L. & Turki, S. Updated checklist
and distribution of large branchiopods (Branchiopoda: Anostraca, Notostraca, Spinicaudata) in Tunisia. _Biogeogr. J. Integr. Biogeogr._ 31, 27–53 (2016). * Mura, G. & Brtek, J. Revised
key to families and genera of the Anostraca with notes on their geographical distribution. _Crustaceana_ 73, 1037–1088 (2000). Google Scholar * Atashbar, B., Agh, N., Van Stappen, G.,
Mertens, J. & Beladjal, L. Combined effect of temperature and salinity on hatching characteristics of three fairy shrimp species (Crustacea: Anostraca). _J. Limnol._ 73, 574–583 (2014).
Google Scholar * Eder, E., Hödl, W. & Gottwald, R. Distribution and phenology of large branchiopods in Austria. _Hydrobiologia_ 359, 13–22 (1997). Google Scholar * Šćiban, M.,
Marković, A., Lukić, D. & Miličić, D. Autumn populations of _Branchinecta orientalis_ G. O. Sars, 1903 and _Chirocephalus diaphanus_ Prevost, 1803 (Crustacea, Branchiopoda) in the
Central European Lowlands (Pannonian Plain, Serbia). _North-West. J. Zool._ 10, 435–437 (2014). Google Scholar * Alonso, M. A survey of the Spanish Euphyllopoda. _Miscelania Zool._ 9,
179–208 (1985). Google Scholar * Petkovski, S. On the presence of the genus _Branchinecta_ Verrill, 1869 (Crustacea, Anostraca) in Yugoslavia. _Hydrobiologia_ 226, 17–27 (1991). Google
Scholar * Dimentman, C. The rainpool ecosystems of Israel: Geographical distribution of freshwater Anostraca (Crustacea). _Israel J. Ecol. Evol._ 30, 1–15 (1981). Google Scholar * Eid, E.
K. New records of large branchiopods from northern Jordan (Crustacea: Branchiopoda). _Zool. Middle East_ 46, 116–117 (2009). Google Scholar * Mura, G., Ozkutuk, S. R., Aygen, C. &
Cottarelli, V. New data on the taxonomy and distribution of anostracan fauna from Turkey. _J. Biol. Res._ 15, 17–23 (2011). Google Scholar * Rogers, D. C., Quinney, D. L., Weaver, J. &
Olesen, J. A new giant species of predatory fairy shrimp from Idaho, USA (Branchiopoda: Anostraca). _J. Crustac. Biol._ 26, 1–12 (2006). Google Scholar * Rodríguez-Flores, P. C.,
Jiménez-Ruiz, Y., Forró, L., Vörös, J. & García-París, M. Non-congruent geographic patterns of genetic divergence across European species of _Branchinecta_ (Anostraca: Branchinectidae).
_Hydrobiologia_ 801, 47–57 (2017). Google Scholar * Atashbar, B., Agh, N., Van Stappen, G. & Beladjal, L. Diversity and distribution patterns of large branchiopods (Crustacea:
Branchiopoda) in temporary pools (Iran). _J. Arid. Environ._ 111, 27–34 (2014). ADS Google Scholar * Belk, D. & Esparza, C. E. Anostraca of the Indian Subcontinent. _Hydrobiologia_
298, 287–293 (1995). Google Scholar * Brtek, J. & Thiéry, A. The geographic distribution of the European Branchiopods (Anostraca, Notostraca, Spinicaudata, Laevicaudata).
_Hydrobiologia_ 298, 263–280 (1995). Google Scholar * Horn, W. & Paul, M. Occurrence and distribution of the Eurasian _Branchinecta orientalis_ (Anostraca) in Central Asia (Northwest
Mongolia, Uvs Nuur Basin) and in other holarctic areas. _Lauterbornia_ 49, 81–91 (2004). Google Scholar * Marrone, F., Alonso, M., Pieri, V., Augugliaro, C. & Stoch, F. The crustacean
fauna of Bayan Onjuul area (Tov Province, Mongolia) (Crustacea: Branchiopoda, Copepoda, Ostracoda). _North West. J. Zool._ 11, 288–295 (2015). Google Scholar * Mura, G. & Takami, G. A.
A contribution to the knowledge of the anostracan fauna of Iran. _Hydrobiologia_ 441, 117–121 (2000). Google Scholar * Naganawa, H. _et al._ Does the dispersal of fairy shrimps
(Branchiopoda, Anostraca) reflect the shifting geographical distribution of freshwaters since the late Mesozoic?. _Limnology_ https://doi.org/10.1007/s10201-019-00589-9 (2019). Article
Google Scholar * Padhye, S. M., Kulkarni, M. R. & Dumont, H. J. Diversity and zoogeography of the fairy shrimps (Branchiopoda: Anostraca) on the Indian subcontinent. _Hydrobiologia_
801, 117–128 (2017). Google Scholar * Petkovski, S. _Taksonomsko-morfološka i zoogeografsko-ekološka studija Anostraca (Crustacea: Branchiopoda) jugoslovenskih zemalja_.
(Prirodno-matematički fakultet, Novi Sad, 1993). * Pretus, J. L. A commented check-list of the Balearic Branchiopoda (Crustacea). _Limnetica_ 6, 157–164 (1990). Google Scholar * van den
Broeck, M., Waterkeyn, A., Rhazi, L. & Brendonck, L. Distribution, coexistence, and decline of Moroccan large branchiopods. _J. Crustacean Biol._ 35, 355–365 (2015). Google Scholar *
Hijmans, R. J., Philips, S., Leathwick, J. & Elith, J. Package ‘dismo’. 9, 1–68 (2017). * R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for
Statistical Computing, Vienna, Austria. http://www.R-project.org/. (2014). * Hijmans, R. J., Cameron, S. E. & Parra, J. L. _Climate Date from Worldclim_ (2004). * Alfonso, G. &
Marrone, F. Branchiopoda Anostraca, Notostraca, Spinicaudata. In _Checklist of the Italian fauna_ (in press). * Defaye, D., Rabet, N. & Thiéry, A. Atlas et bibliographie des crustaces
branchiopodes (Anostraca, Notostraca, Spinicaudata) de France metropolitaine. _Collection patrimoines naturels_ (1998). * Song, H., Buhay, J. E., Whiting, M. F. & Crandall, K. A. Many
species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. _PNAS_ 105, 13486–13491 (2008). ADS CAS PubMed PubMed Central
Google Scholar * Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. _Nucl. Acids Symp. Ser._ 41, 95–98 (1999). CAS Google
Scholar * Aguilar, A. _et al._ High intraspecific genetic divergence in the versatile fairy shrimp _Branchinecta lindahli_ with a comment on cryptic species in the genus Branchinecta
(Crustacea: Anostraca). _Hydrobiologia_ 801, 59–69 (2017). Google Scholar * Jeffery, N. W., Elías-Gutiérrez, M. & Adamowicz, S. J. Species diversity and phylogeographical affinities of
the Branchiopoda (Crustacea) of Churchill, Manitoba, Canada. _PLoS ONE_ 6, e18364 (2011). ADS CAS PubMed PubMed Central Google Scholar * Lanfear, R., Frandsen, P. B., Wright, A. M.,
Senfeld, T. & Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. _Mol. Biol. Evol._ 34,
772–773 (2017). CAS PubMed Google Scholar * Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. _Mol.
Biol. Evol._ 35, 1547–1549 (2018). CAS PubMed PubMed Central Google Scholar * Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST
1.7. _Mol. Biol. Evol._ 29, 1969–1973 (2012). * Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. _Bioinformatics_ 17, 754–755 (2001). CAS PubMed
Google Scholar * Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. _Bioinformatics_ 19, 1572–1574 (2003). CAS PubMed Google Scholar *
Ronquist, F. _et al._ MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. _Syst. Biol._ 61, 539–542 (2012). * Bandelt, H. J., Forster, P.
& Röhl, A. Median-joining networks for inferring intraspecific phylogenies. _Mol. Biol. Evol._ 16, 37–48 (1999). CAS PubMed Google Scholar * Leigh, J. W. & Bryant, D. popart:
Full-feature software for haplotype network construction. _Methods Ecol. Evol._ 6, 1110–1116 (2015). Google Scholar * Xia, X. & Kumar, S. DAMBE7: New and improved tools for data
analysis in molecular biology and evolution. _Mol. Biol. Evol._ 35, 1550–1552 (2018). CAS PubMed PubMed Central Google Scholar * Xia, X. & Lemey, P. Assessing substitution saturation
with DAMBE. In _The phylogenetic Handbook_ 615–630 (Cambridge University Press, 2009). * Xia, X., Xie, Z., Salemi, M., Chen, L. & Wang, Y. An index of substitution saturation and its
application. _Mol. Phylogenet. Evol._ 26, 1–7 (2003). CAS PubMed Google Scholar * Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative
studies of nucleotide sequences. _J. Mol. Evol._ 16, 111–120 (1980). ADS CAS PubMed Google Scholar * Rozas, J. _et al._ DnaSP 6: DNA sequence polymorphism analysis of large data sets.
_Mol. Biol. Evol._ 34, 3299–3302 (2017). CAS PubMed Google Scholar * Nychka, D. _et al. fields: Tools for Spatial Data_ (2020). * Oksanen, J. _et al._ vegan: Community ecology package. –
R package ver. 2.0-4. http://CRAN.R-project.org/package=vegan. (2012). Download references ACKNOWLEDGEMENTS The work was supported by the Interreg V-A Austria-Hungary program of the European
Regional Development Fund (“Vogelwarte Madárvárta 2”) and ÖAD (Erasmus+ internship mobility grant). D.L. was a recipient of the DOC fellowship of the Austrian Academy of Sciences and
Nationalparks Austria research scholarship at the WasserCluster Lunz and is currently employed on an FWF project—FWF P 32714. Z. H. acknowledges support by the NKFIH OTKA FK-132095,
NKFIH-471-3/2021, and the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The work of M. M. was supported by grant no. 2017/27/B/NZ8/01056 from the National Science
Centre, Poland. Authors thank Lake Neusiedl Biological Station, Thomas Zechmeister, Richard Haider, Jordi Sala, Raquel Ortells, Priscila Pons, Bart Hellemans, Csenge Póda, Adam Petrusek and
Luca Vecchioni for their help in field work, sample collection, work in the molecular lab or performing part of the analyses. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * WasserCluster
Lunz, Lunz am See, Austria Dunja Lukić, Csaba F. Vad, Robert Ptacnik & Zsófia Horváth * Research Department for Limnology, University of Innsbruck, Mondsee, Austria Dunja Lukić *
Laboratory of Animal Ecology, Global Change and Sustainable Development, KU Leuven, Leuven, Belgium Tom Pinceel & Luc Brendonck * Community Ecology Laboratory, Department of Biology,
Vrije Universiteit Brussel (VUB), Brussels, Belgium Tom Pinceel * Centre for Environmental Management, University of the Free State, Bloemfontein, South Africa Tom Pinceel * Department of
Biological, Chemical and Pharmaceutical Sciences, University of Palermo, Palermo, Italy Federico Marrone * Department of Genetics and Biosystematics, University of Gdańsk, Gdańsk, Poland
Monika Mioduchowska * Department of Marine Plankton Research, University of Gdańsk, Gdynia, Poland Monika Mioduchowska * Institute of Aquatic Ecology, Centre for Ecological Research,
Budapest, Hungary Csaba F. Vad & Zsófia Horváth * Laboratory of Aquatic Ecology, Evolution and Conservation, KU Leuven, Leuven, Belgium Csaba F. Vad & Zsófia Horváth * Water Research
Group, Unit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa Luc Brendonck * Department of Invertebrate Zoology and Hydrobiology, Faculty of
Biology and Environmental Protection, University of Lodz, Banacha 12/16, 90-237, Lodz, Poland Monika Mioduchowska Authors * Dunja Lukić View author publications You can also search for this
author inPubMed Google Scholar * Tom Pinceel View author publications You can also search for this author inPubMed Google Scholar * Federico Marrone View author publications You can also
search for this author inPubMed Google Scholar * Monika Mioduchowska View author publications You can also search for this author inPubMed Google Scholar * Csaba F. Vad View author
publications You can also search for this author inPubMed Google Scholar * Luc Brendonck View author publications You can also search for this author inPubMed Google Scholar * Robert Ptacnik
View author publications You can also search for this author inPubMed Google Scholar * Zsófia Horváth View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS D.L., Zs.H., Cs.V. and R.P. conceived this study. D.L. and M.M. performed the lab work with help from T.P. and L.B. D.L. analysed the data with the help of Zs.H., T.P., M.M.
and F.M. D.L. and Zs.H. wrote the first version of the manuscript, after which all authors contributed to improving the manuscript. CORRESPONDING AUTHOR Correspondence to Dunja Lukić. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lukić, D., Pinceel, T., Marrone, F. _et al._ Pleistocene allopatric
differentiation followed by recent range expansion explains the distribution and molecular diversity of two congeneric crustacean species in the Palaearctic. _Sci Rep_ 11, 22866 (2021).
https://doi.org/10.1038/s41598-021-02164-8 Download citation * Received: 20 July 2021 * Accepted: 03 November 2021 * Published: 24 November 2021 * DOI:
https://doi.org/10.1038/s41598-021-02164-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
