Rapamycin-encapsulated nanoparticle delivery in polycystic kidney disease mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Rapamycin slows cystogenesis in murine models of polycystic kidney disease (PKD) but failed in clinical trials, potentially due to insufficient drug dosing. To improve drug efficiency
without increasing dose, kidney-specific drug delivery may be used. Mesoscale nanoparticles (MNP) selectively target the proximal tubules in rodents. We explored whether MNPs can target
cystic kidney tubules and whether rapamycin-encapsulated-MNPs (RapaMNPs) can slow cyst growth in Pkd1 knockout (KO) mice. MNP was intravenously administered in adult Pkd1KO mice. Serum and
organs were harvested after 8, 24, 48 or 72 h to measure MNP localization, mTOR levels, and rapamycin concentration. Pkd1KO mice were then injected bi-weekly for 6 weeks with RapaMNP,
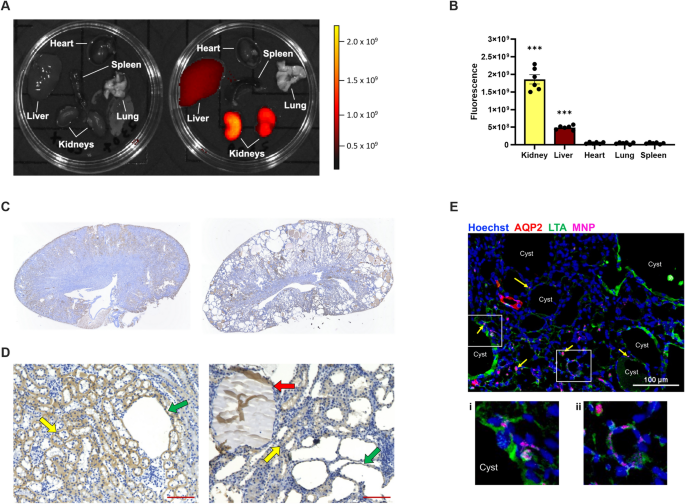
rapamycin, or vehicle to determine drug efficacy on kidney cyst growth. Single MNP injections lead to kidney-preferential accumulation over other organs, specifically in tubules and cysts.
Likewise, one RapaMNP injection resulted in higher drug delivery to the kidney compared to the liver, and displayed sustained mTOR inhibition. Bi-weekly injections with RapaMNP, rapamycin or
vehicle for 6 weeks resulted in inconsistent mTOR inhibition and little change in cyst index, however. MNPs serve as an effective short-term, kidney-specific delivery system, but long-term
RapaMNP failed to slow cyst progression in Pkd1KO mice.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic kidney disorder, with over 50% of patients eventually developing end stage kidney disease. Most ADPKD patients
carry mutations on the PKD1 or PKD2 genes, though kidney cysts appear earlier and progress more rapidly with PKD1 mutations1. Currently, tolvaptan is the only Food and Drug Administration
(FDA) approved drug to treat PKD2,3. However, tolvaptan results in polyuria and constant thirst. It also carries a risk for liver dysfunction, requiring patients to undergo routine bloodwork
for surveillance. A well-known pathway that stimulates proliferation and cystogenesis in ADPKD is the mammalian target of rapamycin (mTOR) pathway4,5. Although mTOR inhibitors sirolimus and
everolimus were shown to slow cyst growth in PKD rodents, they did not slow ADPKD in clinical trials6,7. This may be, in part, a result of dose reduction due to adverse effects, leading to
inadequate kidney mTOR inhibition8,9. To overcome this limitation, and provide a safer, more effective dose, kidney-specific drug delivery could be utilized to circumvent drug metabolism by
the liver.
Nanoparticles (NP), whose bio-distribution is dictated by surface material and size, have recently shown potential applications in diagnostics, imaging, and drug delivery, including cancer
treatment10. FDA-approved poly lactic-co-glycolic acid (PLGA) polymers are physically strong, biocompatible, and biodegradable11. Unsurprisingly, these qualities make PLGA polymers an ideal
surface material for drug-encapsulated NPs. To specifically target the kidney, particles must be large enough not to be quickly cleared from the body upon renal tubule bio-distribution (> 2
nm)12,13, yet small enough not to accumulate in the liver, spleen14, or lungs15 (
