The corticomotor projection to liminally-contractable forearm muscles in chronic spinal cord injury: a transcranial magnetic stimulation study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT STUDY DESIGN: A cross-sectional study in chronic spinal cord injury with cervical lesions (cSCI). OBJECTIVE: To determine the corticomotor projection and motor cortex organization
of paralyzed forearm muscles that presented only liminal voluntary activation. SETTING: Burke Medical Research Institute, White Plains, NY, USA. METHODS: We identified ten people with
chronic SCI who had a wrist flexor or extensor muscle with a motor power (MP) of 1 over 5. We recorded motor evoked potentials (MEPs) to transcranial magnetic stimulation (TMS) over the
primary motor cortex of the hemisphere contralateral to the target muscle. We measured resting motor threshold (RMT), corticomotor latency (LTY), MEP amplitude (AMP) and performed cortical
motor mapping to determine the optimal site (OPT) and map area (AREA). Results were compared with the data from 18 controls. RESULTS: A MEP in the target muscle was observed for all cSCI
cases. LTY was normal, while corticomotor excitability (as determined by RMT and AMP) was reduced in about half of the group. The OPT site of the motor maps was within control range for all
cSCI cases, while AREA was reduced in three cases. CONCLUSIONS: Corticomotor conduction and cortical topography were appreciably normal despite only liminal activation of the target muscle
with voluntary effort. Muscles with these characteristics may benefit from a targeted rehabilitation program even in the chronic phase after SCI. SIMILAR CONTENT BEING VIEWED BY OTHERS
CHARACTERIZING NEUROLOGICAL STATUS IN INDIVIDUALS WITH TETRAPLEGIA USING TRANSCUTANEOUS SPINAL STIMULATION Article Open access 06 December 2023 NON-INVASIVE SPINAL CORD ELECTRICAL
STIMULATION FOR ARM AND HAND FUNCTION IN CHRONIC TETRAPLEGIA: A SAFETY AND EFFICACY TRIAL Article Open access 20 May 2024 FUNCTIONAL ELECTRICAL STIMULATION THERAPY FOR UPPER EXTREMITY
REHABILITATION FOLLOWING SPINAL CORD INJURY: A PILOT STUDY Article 01 April 2023 INTRODUCTION A cervical spinal cord injury (cSCI) can result in paralysis or severe motor deficits in the
upper and lower extremities that can have a devastating impact on independence and quality of life.1 In the upper extremities, recovery of function in the wrist flexors and extensors is
important as it can lead to improvements in arm and hand function.2, 3 The American Spinal Injury Association (ASIA) Impairment Scale (AIS) is widely used to classify impairment (motor and
sensory) after spinal cord injury. For the assessment of upper limb motor power, the AIS scale uses the Medical Research Council (MRC) scale for motor power (MP) and pools this for 5
functionally relevant movements (including wrist extension). The MRC scale ranges from 0 to 5, with a MP of 1 defined as a trace of muscle contraction or fasciculation, but no overt movement
about the joint. We recently described a cSCI case study in which corticospinal conduction to the wrist flexors and extensors was remarkably preserved, even though wrist MP was only 1 over
5.4 Using transcranial magnetic stimulation (TMS), we reported motor evoked potentials (MEPs) of normal amplitude (AMP) and latency for these wrist muscles. This observation suggested that
supraspinal motor-related networks could be implicated in functional weakness after cSCI.5, 6 However, the changes that might occur in these networks in response to cSCI are not well
understood. It is known that spinal cord injury can result in cortical atrophy7, 8, 9 and there is evidence for cortical reorganisation from functional magnetic resonance imaging (fMRI),
positron emission tomography (PET) and electroencephalographic (EEG) studies.10, 11 TMS can be used to map the corticomotor projection from primary motor cortex (M1) to a target muscle12 and
provides another opportunity to investigate cortical reorganisation. While TMS mapping has been used to identify reorganisation of muscles innervated above the level of the lesion,10, 13
there have been no such studies of severely impaired muscles at the level of the lesion. In the present study, we have measured corticomotor conduction and performed TMS mapping in a group
of 10 people with cSCI who presented with muscle strength in a forearm muscle of 1 over 5. Our aims were to determine how corticomotor conduction was affected in people with a MP of 1/5, and
to determine whether the cortical maps were normal or showed signs of reorganisation. METHODS PARTICIPANTS Ten people with cSCI volunteered to participate in the study (Table 1). All met
the following inclusion criteria: age 18–70 years, chronic injury (>1 year after injury), cervical injury level, traumatic lesion, motor complete or incomplete, MP of wrist extensor or
flexor muscles 1/5, no evidence of trauma-related brain injury, no contraindications for TMS, no history of seizure and medically stable. A second investigator independently confirmed the MP
score. All cSCI participants were right-handed prior to injury and remained so thereafter. None of the participants had a history of neurological or psychiatric disorders. Eighteen
right-handed healthy participants (24–51 years of age, 8 male) without a history of neurological or psychiatric illness were recruited as a control group. The Institutional Review Board of
the Burke Rehabilitation Hospital approved the experimental protocol, and all subjects gave written informed consent prior the experiment. MOTOR POWER The MP of the Extensor Carpi Radialis
(ECR) and Flexor Carpi Radialis (FCR) muscles on the left and right sides was scored independently by two neurologists. The muscle identified by both scorers as having a MP of 1 was the
target muscle for the remainder of the study. EMG RECORDING Bipolar surface electromyography (EMG) electrodes (1 cm diameter, 2 cm inter-pole distance, × 1000 gain, band-pass filter 20–400
Hz) were placed over the belly of the target muscle. EMG activity was recorded by Biometrics electromyography (Biometrics Ltd, UK), and signals were fed into CED 1401 using Spike 2.6 for
further off-line analysis. During the experiments, EMG activity was continuously monitored with visual feedback to ensure complete muscle relaxation. An estimate of maximum voluntary
contraction (MVC) in the cSCI group was obtained by recording short periods of EMG, while the participant attempted a maximum muscle contraction. Three 0.5 s epochs of EMG were acquired, and
the average root-mean-square EMG calculated. Three 0.5 s epochs of resting EMG were also acquired as an estimate of signal noise. TMS measurements were taken for the ECR and FCR muscles on
the left and right sides in the 18 controls. TRANSCRANIAL MAGNETIC STIMULATION (TMS) SCI participants were seated in their own wheelchairs and wore a snugly-fitting pre-marked cap with sites
marked in spacings of 1 cm in latitude and 2 cm in longitude in relation to the vertex and inter-aural line.12, 14 A figure-of-eight coil (DB-80, Tonika Elektronik A/S, DK-3520, Denmark)
was connected to a MagPro magnetic stimulator (MagVenture, Farum, Denmark). The coil was manually positioned on the scalp over the expected location of the contralateral primary motor cortex
(M1), with the handle pointing backwards at an angle of 45 degrees to the midline.15 The optimal site was established by a search pattern at suprathreshold intensity to determine the
location yielding the largest MEPs. Resting motor threshold (RMT) was measured at this site and was defined as the minimum intensity (2% steps in stimulator output) required to elicit at
least 3 MEPs with a peak-peak AMP >50 μV in 5 consecutive trials.16, 17 For the remainder of the experiment, TMS intensity was set to 1.2 × RMT. Twenty MEPs were recorded at the optimal
site at this intensity. MEP peak-peak AMP and MEP onset latency (LTY) were determined from these data by manual cursoring and averaged for each muscle. MOTOR CORTEX MAPPING Five stimuli were
delivered at a frequency of 0.2 Hz at each stimulation site starting at the optimal site and then successively stimulating adjacent sites until no MEP was found (<50 μV AMP). To maintain
consistency in coil orientation as it was repositioned over the scalp for mapping, the coil was held in the parasagittal plane. Maps were generated by fitting a continuous function to the
mean peak-to-peak MEP AMP at each scalp site (Wilson 1993). The latitude (LAT) and longitude (LONG) at which the map had its maximum AMP was determined. LAT was expressed as the distance in
centimeters from the vertex, and LONG the distance in centimeters anterior (positive) or posterior (negative) to the inter-aural line. To avoid biasing map area (AREA) by small MEPs on the
periphery, AREA was calculated from the region of the map with an AMP >1/8 of maximum. STATISTICAL ANALYSIS A Wilcoxon rank-sum was used to test for an effect of SIDE (left, right) and
MUSCLE (ECR, FCR). A two-way ANOVA was used to assess for an interaction between SIDE and MUSCLE. From the control data, 95% confidence intervals were determined for each parameter (RMT,
AMP, LTY, LAT, LONG, AREA). The lower limit (LL) was 2 s.d. below the mean and the upper limit (UL) was 2 s.d. above the mean. The AMP data was skewed and log transforms were used to
establish LL and UL. The SCI data for each participant was compared with control ranges. RESULTS SCI subject’s characteristics are given in Table 1. The mean age was 42.4±16.5 years (s.d.;
range 17–70 years of age; 7 M, 3 F). The mean period following injury was 6.1±8.2 years (range 1.8–29 years). Eight of the SCI group had incomplete lesions and two had complete lesions. Two
subjects were classified as motor-sensory complete (AIS A), five as motor complete but sensory incomplete (AIS B), and three as motor and sensory incomplete (one as AIS C). All participants
had severe upper and lower limb impairment. Across the SCI group, the target muscles that had a MP of 1 were evenly split between left and right sides (five cases each) and ECR and FCR (also
five cases for each). The mean age of the control group was 36.1±6.8 (s.d., range 24–51 years). A Wilcoxon rank-sum test revealed no effect of SIDE (for all parameters) but an effect of
MUSCLE for most parameters (_P_<0.05). As a result, the data was pooled for the left and right sides and control ranges (LL, UL) established separately for the ECR and FCR (see Table 2).
For the SCI group, an analysis using two-way Anova revealed there were no effects for SIDE, MUSCLE or SIDE x MUSCLE. Correspondingly, the SCI data from either side (left or right) was
compared with the control ranges for ECR and FCR separately. CORTICOMOTOR EXCITABILITY AND CONDUCTION A MEP was observed for all cases in the SCI group. The LTY was within control range for
all but one of the SCI cases. For all five SCI cases, in whom the ECR was studied, RMT and AMP were outside the control ranges and there was a significant difference between this SCI
subgroup and controls (_t_-test, _P_<0.001 and _P_=0.003, respectively). For the five cases in whom the FCR was studied, RMT and AMP were within the control ranges for all but one case
and there was a significant difference between this subgroup and controls for RMT (_P_<0.02). There was no significant difference between ECR or FCR for RMT or for AMP (_P_=0.7 and
_P_=0.4, respectively). The RMS ranged from 0.008 to 0.017 mV for ECR and 0.007 to 0.015 mV for FCR (mean for combined muscles 0.011±0.004 mV). Mean resting RMS was 0.007±0.002 μV. There was
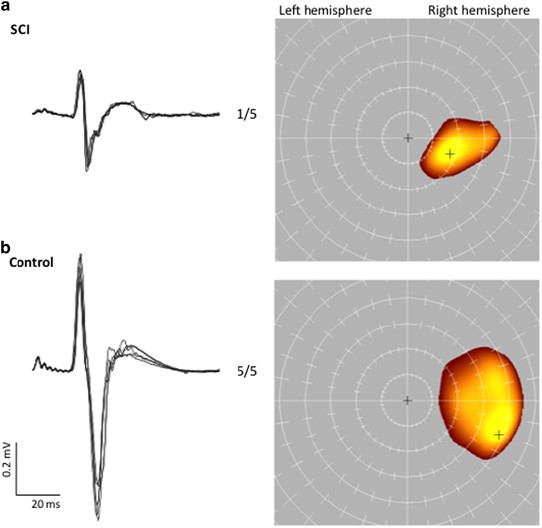
no correlation between RMS during an attempted MVC and AMP (_P_=0.22). CORTICOMOTOR MAPPING The TMS maps could be obtained for all SCI cases. Figure 1 gives MEP waveforms and maps for a
representative case from the SCI group and the control group. Table 2 gives the control ranges for each parameter and Table 3 provides the results for each case in the SCI group. The LAT and
LONG were within the control range for all SCI cases (Figure 2). The AREA was reduced in the FCR for three cases (two marginally). DISCUSSION It was possible to determine a RMT, record a
MEP and measure a motor map for a forearm muscle in all cases from this group of chronic cervical SCI. The unifying feature in the group was the selection of a target muscle with a MP of
1/5. We showed that corticomotor conduction through the level of the spinal injury is remarkably patent and that the topographic origins of this projection in M1 are normal. The results
indicate that in the chronic phase after injury, and in muscles with only liminal voluntary activation, there can remain a significant neurophysiological substrate that could be a target for
rehabilitation. We have previously reported that corticomotor conduction can be within control values in a case study of chronic cervical SCI.4, 18 The present study generalises this
finding by showing that it is not rare to find patent corticomotor conduction in muscles with only liminal levels of voluntary activation in the chronic phase after SCI. Corticomotor
conduction time was within control range for all but one SCI case that was marginally delayed. This is in contrast to some previous studies that indicate conduction through the lesion is
often delayed.19, 20, 21 However, spinal cord injury is expected to have diverse effects on corticospinal conduction depending on the nature of the lesion. In our cSCI group, selected to
have a degree of commonality (chronic and stable, cervical lesions, forearm wrist extensors/flexors, MP=1), we find that the fast-conducting monosynaptic pathway to the target muscles can be
relatively normal. While RMT was increased and AMP decreased in about half of the cases, in many these data were not greatly outside of control range. Across the group, we mostly observed
these abnormalities for the ECR rather than the FCR muscle. In the control group, we found inter-muscle differences, with the ECR having a lower RMT and higher AMP than the FCR. This
difference was not seen in the SCI group, presumably because RMT was increased and AMP reduced mostly just for the ECR muscle. The reason for this is not certain; however, it could be
related to differences in the corticospinal projection to wrist flexor and extensor muscles. It is known that wrist extension can be severely affected in SCI22 and the wrist extensors are
target muscles in the AIS scoring system.23 The retention of corticospinal conduction through the lesion is consistent with anatomical studies that show there are usually spared axons across
a lesion.24, 25, 26 However, less is known about the corticomotor pathways these axons serve. The TMS mapping data suggest that the spared axons that give rise to the MEP originate from the
expected forearm representation in M1. It is known that the cerebral cortex is remarkably adaptable, and that cortical and subcortical lesions can lead to functional reorganisation.27, 28,
29 Previous studies of cortical reorganisation after SCI have mostly employed functional imaging techniques such as functional MRI and PET.11 These studies require voluntary activation of a
target limb, often targeting muscles proximally to the lesion, which can recruit multiple brain regions and involve polysynaptic pathways not accessible to TMS. The present study was
performed in forearm muscles with MP=1 and indicated that the corticospinal neurons giving rise to the MEP in these muscles had a normal cortical topography. We cannot say whether this would
be true in higher-functioning proximal muscles, where reorganisation might take place as a result of use-dependent plasticity, or in muscles of the lower extremity. Perhaps the most
intriguing finding is that even for those muscles with normal corticomotor conduction and cortical topography, the muscles could only be liminally activated under voluntary control, even
when participants were asked to do a maximal effort. The underlying cause of this functional paralysis is not certain and may perhaps involve a learned disuse. If so, the present results
increase confidence that clinically meaningful improvement in function might be achieved with a suitable rehabilitation program. The objective data of corticomotor conduction, such as
provided by TMS, may also increase the likelihood of success, if communicated to participants. In conclusion, in our cSCI group, targeting a forearm muscle with MP=1/5, corticomotor
conduction and cortical topography were appreciably normal despite only liminal activation with voluntary effort. This suggests that muscles with these characteristics may benefit from a
targeted rehabilitation program even in the chronic phase after SCI. In the absence of TMS, our data suggests that further rehabilitation of muscles with a MP of 1/5 might be indicated.
Demonstration of a good cortical-motor connection by TMS may be a fillip to people struggling with recovery of weakly activated muscles after SCI. REFERENCES * Welch RD, Lobley SJ,
O'Sullivan SB, Freed MM . Functional independence in quadriplegia: critical levels. _Arch Phys Med Rehabil_ 1986; 67: 235–240. CAS PubMed Google Scholar * Ditunno JF Jr, Sipski ML,
Posuniak EA, Chen YT, Staas WE Jr, Herbison GJ . Wrist extensor recovery in traumatic quadriplegia. _Arch Phys Med Rehabil_ 1987; 68 (5 Pt 1): 287–290. PubMed Google Scholar * Hanson RW,
Franklin MR . Sexual loss in relation to other functional losses for spinal cord injured males. _Arch Phys Med Rehabil_ 1976; 57: 291–293. CAS PubMed Google Scholar * Edwards DJ, Cortes
M, Thickbroom GW, Rykman A, Pascual-Leone A, Volpe BT . Preserved corticospinal conduction without voluntary movement after spinal cord injury. _Spinal Cord_ 2013; 51: 765–767. Article CAS
PubMed PubMed Central Google Scholar * Curt A, Dietz V . Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. _Spinal Cord_ 1999;
37: 157–165. Article CAS PubMed Google Scholar * Iseli E, Cavigelli A, Dietz V, Curt A . Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and
electrophysiological evaluation. _J Neurol Neurosurg Psychiatry_ 1999; 67: 567–571. Article CAS PubMed PubMed Central Google Scholar * Jurkiewicz MT, Crawley AP, Verrier MC, Fehlings
MG, Mikulis DJ . Somatosensory cortical atrophy after spinal cord injury: a voxel-based morphometry study. _Neurology_ 2006; 66: 762–764. Article CAS PubMed Google Scholar * Nie B, Chen
K, Zhao S, Liu J, Gu X, Yao Q _et al_. A rat brain MRI template with digital stereotaxic atlas of fine anatomical delineations in paxinosspace and its automated application in voxel-wise
analysis. _Hum Brain Mapp_ 2013; 34: 1306–1318. Article PubMed Google Scholar * Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT _et al_. Rewiring of hindlimb corticospinal
neurons after spinal cord injury. _Nat Neurosci._ 2010; 13: 97–104. Article CAS PubMed Google Scholar * Lotze M, Laubis-Herrmann U, Topka H . Combination of TMS and fMRI reveals a
specific pattern of reorganization in M1 in patients after complete spinal cord injury. _Restor Neurol Neurosci_ 2006; 24: 97–107. CAS PubMed Google Scholar * Kokotilo KJ, Eng JJ, Curt A
. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. _J Neurotrauma_ 2009; 26: 2113–2126. Article PubMed Google Scholar * Wilson SA,
Thickbroom GW, Mastaglia FL . Transcranial magnetic stimulation mapping of the motor cortex in normal subjects. the representation of two intrinsic hand muscles. _J Neurol Sci_ 1993; 118:
134–144. Article CAS PubMed Google Scholar * Freund P, Rothwell J, Craggs M, Thompson AJ, Bestmann S . Corticomotor representation to a human forearm muscle changes following cervical
spinal cord injury. _Eur J Neurosci_ 2011; 34: 1839–1846. Article PubMed Google Scholar * Thickbroom GW, Byrnes ML, Mastaglia FL . Methodology and application of TMS mapping.
_Electroencephalogr Clin Neurophysiol Suppl_ 1999; 51: 48–54. CAS PubMed Google Scholar * Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M . Optimal focal transcranial
magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. _J Clin Neurophysiol_ 1992; 9: 132–136. Article CAS
PubMed Google Scholar * Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of
transcranial magnetic stimulation in clinical practice and research. _Clin Neurophysiol_ 2009; 120: 2008–2039. Article PubMed PubMed Central Google Scholar * Rothwell JC, Hallett M,
Berardelli A, Eisen A, Rossini P, Paulus W . Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. _Electroencephalogr Clin Neurophysiol
Suppl_ 1999; 52: 97–103. CAS PubMed Google Scholar * Edwards DJ, Cortes M, Thickbroom GW, Rykman A, Pascual-Leone A, Volpe BT . Reply: evidence against volume conduction to explain normal
MEPs in muscles with low motor power in SCI. _Spinal Cord_ 2014; 52: 718. Article CAS PubMed PubMed Central Google Scholar * Calancie B, Alexeeva N, Broton JG, Suys S, Hall A, Klose KJ
. Distribution and latency of muscle responses to transcranial magnetic stimulation of motor cortex after spinal cord injury in humans. _J Neurotrauma_ 1999; 16: 49–67. Article CAS PubMed
Google Scholar * Li K, Atkinson D, Boakye M, Tolfo CZ, Aslan S, Green M _et al_. Quantitative and sensitive assessment of neurophysiological status after human spinal cord injury. _J
Neurosurg Spine_ 2012; 17: 77–86. Article PubMed Google Scholar * Awad BI, Carmody MA, Zhang X, Lin VW, Steinmetz MP . Transcranial magnetic stimulation after spinal cord injury. _World
Neurosurg_ 2015; 83: 232–235. Article PubMed Google Scholar * Chye L, Nosaka K, Murray L, Edwards D, Thickbroom G . Corticomotor excitability of wrist flexor and extensor muscles during
active and passive movement. _Hum Mov Sci_ 2010; 29: 494–501. Article PubMed Google Scholar * Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF _et al_. International
Campaign for Cures of Spinal Cord Injury Paralysis. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome
measures. _Spinal Cord_ 2007; 45: 206–221. Article CAS PubMed Google Scholar * Bunge RP, Puckett WR, Hiester ED . Observations on the pathology of several types of human spinal cord
injury, with emphasis on the astrocyte response to penetrating injuries. _Adv Neurol_ 1997; 72: 305–315. CAS PubMed Google Scholar * Kakulas BA . Neuropathology: the foundation for new
treatments in spinal cord injury. _Spinal Cord_ 2004; 42: 549–563. Article CAS PubMed Google Scholar * Tansey KE, McKay WB, Kakulas BA . Restorative neurology: consideration of the new
anatomy and physiology of the injured nervous system. _Clin Neurol Neurosurg_ 2012; 114: 436–440. Article PubMed Google Scholar * Hou J, Xiang Z, Yan R, Zhao M, Wu Y, Zhong J _et al_.
Motor recovery at 6 months after admission is related to structural and functional reorganization of the spine and brain in patients with spinal cord injury. _Hum Brain Mapp_ 2016; 37:
2195–2209. Article PubMed PubMed Central Google Scholar * Sydekum E, Ghosh A, Gullo M, Baltes C, Schwab M, Rudin M . Rapid functional reorganization of the forelimb cortical
representation after thoracic spinal cord injury in adult rats. _Neuroimage_ 2014; 87: 72–79. Article PubMed Google Scholar * Tandon S, Kambi N, Mohammed H, Jain N, . Complete
reorganization of the motor cortex of adult rats following long-term spinal cord injuries. _Eur J Neurosci_ 2013; 38: 2271–2279. Article PubMed Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Non-invasive Brain Stimulation and Human Motor Control Laboratory, Burke Medical Research Institute, White Plains, NY, USA M Cortes, G W Thickbroom, A
Rykman & D J Edwards * Department of Neurology, Cornell University, New York, NY, USA M Cortes & D J Edwards * Neurology Department, EMG and Motor Control Unit, Hospital Clinic,
Universitat de Barcelona, Barcelona, Spain M Cortes & J Valls-Sole * Department of Rehabilitation Medicine, Cornell University, New York, NY, USA G W Thickbroom * Department of
Epidemiology, Cornell University, New York, NY, USA J Elder * Division of Cognitive Neurology, Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center,
Harvard Medical School, Boston, MA, USA A Pascual-Leone & D J Edwards * Department of Neurology, Institut Universitari de Neurorehabilitacio Guttmann, Universitat Autonoma, Barcelona,
Spain A Pascual-Leone Authors * M Cortes View author publications You can also search for this author inPubMed Google Scholar * G W Thickbroom View author publications You can also search
for this author inPubMed Google Scholar * J Elder View author publications You can also search for this author inPubMed Google Scholar * A Rykman View author publications You can also search
for this author inPubMed Google Scholar * J Valls-Sole View author publications You can also search for this author inPubMed Google Scholar * A Pascual-Leone View author publications You
can also search for this author inPubMed Google Scholar * D J Edwards View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to M Cortes. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION DATA ARCHIVING There were no data to deposit. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cortes, M., Thickbroom, G., Elder, J. _et al._ The corticomotor projection to liminally-contractable forearm muscles in chronic
spinal cord injury: a transcranial magnetic stimulation study. _Spinal Cord_ 55, 362–366 (2017). https://doi.org/10.1038/sc.2016.161 Download citation * Received: 29 March 2016 * Revised:
22 July 2016 * Accepted: 26 September 2016 * Published: 20 December 2016 * Issue Date: April 2017 * DOI: https://doi.org/10.1038/sc.2016.161 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative
