Differential effects of propranolol on conditioned hyperactivity and locomotor sensitization induced by morphine in rats
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT According to memory reconsolidation theory, when long-term memory is reactivated by relevant clues, the memory traces become labile, which can be altered by pharmacological
manipulations. Accumulating evidence reveals that memory related to drug abuse can be erased by disrupting reconsolidation process. We used an animal model that could simultaneously measure
conditioned hyperactivity and locomotor sensitization induced by morphine. β-adrenoceptor antagonist propranolol or saline were administered following conditioned stimuli (CS) or a small
dose of morphine reactivation. The results showed that the conditioned hyperactivity could be disrupted by propranolol treatment following CS reactivation. However, the expression of
locomotor sensitization could not be disrupted by propranolol administration following CS or morphine reactivation. Furthermore, morphine injection and propranolol intervention enhanced the
locomotor sensitization effect. These data suggest that blocking the reconsolidation process can disrupt the conditioned hyperactivity induced by environmental cues associated with morphine
treatment, but not morphine-induced locomotor sensitization. SIMILAR CONTENT BEING VIEWED BY OTHERS ROLE OF DOPAMINE D1 RECEPTOR IN THE MODULATION OF MEMORY CONSOLIDATION BY PASSIVE AND
SELF-ADMINISTERED HEROIN AND ASSOCIATED CONDITIONED STIMULI Article Open access 03 August 2023 THE ANXIOGENIC DRUG YOHIMBINE IS A REINFORCER IN MALE AND FEMALE RATS Article 17 September 2024
THE EFFECTS OF PASSIVE AND ACTIVE ADMINISTRATION OF HEROIN, AND ASSOCIATED CONDITIONED STIMULI, ON CONSOLIDATION OF OBJECT MEMORY Article Open access 27 November 2022 INTRODUCTION Drug
addiction is a chronic relapsing disorder characterized by compulsive drug seeking and use. Over 80% of addicts relapse to drug seeking and use after a period of withdrawal and abstinence1.
For instance, cocaine abusers exhibit strong conditioned craving when they are presented with stimuli previously associated to cocaine use in a laboratory setting2. Consequently, there are
two major aims in preclinical research: one is to clarify the behavioral, environmental and neural mechanisms underlying relapse and the other is to discover medications that can prevent
relapse. A major contributor to relapse is exposure to environmental stimuli that have previously been associated regularly with drugs3. Many studies have shown that neutral clues can
acquire excitatory locomotor (hyperactivity) effect in a drug free state when drug administration is repeatedly paired with those clues4,5,6,7. Locomotor sensitization refers to a
progressive and persistent increase in the psychomotor activating effects of drugs (e.g. opioids and psychostimulants), which often occurs when drugs of abuse are given repeatedly and
intermittently4,8,9,10. Sensitization-related neuroplasticity in brain reward systems may contribute to addiction8,9,10. The process of previously consolidated memories being recalled and
actively consolidated is defined as the memory reconsolidation. During this process, memory traces become labile and can be altered by various pharmacological manipulations11,12,13.
Increasing studies have begun to reveal that memory reconsolidation is mediated by various neural events, including receptors14,15, signal transduction pathways16,17 and proteins18,19. Using
conditioned place preference (CPP)20,21,22,23,24, self-administration25 and conditioned approach26 paradigms, it has been demonstrated that disruption of reconsolidation could impair the
expression of drug-associated memory, which suggests that such a technique to target the reconsolidation process could be a prospective treatment for drug addiction. Evidence shows that the
noradrenergic system is critically involved in memory reconsolidation. For example, the administration of β-adrenoceptor antagonist propranolol after the reactivation of cocaine-27,28 or
morphine-29,30,31 induced CPP impairs the conditioning response. There are only a few studies that have examined the reconsolidation of memories underlying drug-induced locomotor
sensitization. Bernardi et al32 have reported that systemic anisomycin treatment given immediately after a reactivation session in which rats are put into the cocaine-associated context
blocks cocaine-induced locomotor sensitization. However, Valjent et al24 found no effect with anisomycin using a similar paradigm. Exposure of animals to drug-conditioned context/cues (CS)
in the absence of drug administration (unconditioned stimuli, US) has frequently been used to reactivate drug-context association21,27. However, some researchers suggest that reactivation
requires the re-experience of a conditioning session and it does not occur after contextual or drug exposure alone22,24,33,34. It is important to efficiently and effectively reactivate drug
related memory in order to fully assess the reconsolidation-interfering effect of propranolol treatment. Therefore, in the current study morphine treatment-related memory was reactivated in
two consecutive days in which propranolol was administered immediately after CS- or US- primed reinstatement sessions. The goal of this study was to test the feasibility of
propranolol's disrupting effect on the reconsolidation of conditioned hyperactivity and locomotor sensitization induced by morphine. We also tested the effect of different retrieval
types in reactivating memory reconsolidation underlining locomotor sensitization. RESULTS The group assignment, timeline and treatment for the experiments were shown in Table 1. Briefly, the
experimental procedure consists of three sessions, the acquisition of conditioned hyperactivity and locomotor sensitization, reactivation and intervention, conditioned hyperactivity and
locomotor sensitization test. THE ACQUISITION OF CONDITIONED HYPERACTIVITY AND LOCOMOTOR SENSITIZATION During the conditioning sessions, conditioned hyperactivity is defined as the
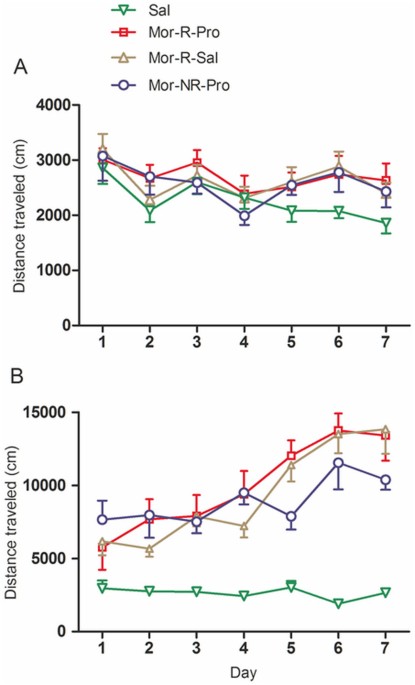
locomotion during the 20 min prior to drug administration. As shown in Fig. 1A, repeated two-way ANOVA showed a significant main effect of days (F6, 222 = 6.82; p < 0.001), but the
interaction of group × days (F18,222 = 0.853; p = 0.67) and the effect of groups (F3, 37 = 1.33; p = 0.28) were not significant. Rats received morphine (Mor-R-Sal, Mor-R-Pro and Mor-NR-Pro
groups) showed higher locomotion than rats received saline (Sal group) during the conditioning sessions. However, there were no statistically differences among the groups that received
morphine (p > 0.05). The locomotor sensitization to morphine is defined as the progressive increase in locomotion observed after intermittent administrations of morphine. As shown in Fig.
1B, during the daily 120-min locomotor recording, statistical analysis revealed a significant effect of days (F6,222 = 27.22; p < 0.001), an interaction of group × days (F18,222 = 6.748;
p < 0.001) and an effect of groups (F3, 37 = 17.129; p < 0.001). Rats given morphine (Mor-R-Sal, Mor-R-Pro and Mor-NR-Pro groups) during the training sessions showed significantly
higher locomotion level than those received saline (p < 0.05 for every session). Moreover, although there were fluctuations during the training session, their locomotion level on Day 7
was significantly higher than that on Day 1 (p = 0.003, p = 0.001 and p = 0.04 for group Mor-R-Sal, Mor-R-Pro and Mor-NR-Pro), which demonstrated the progressive development of morphine
locomotor sensitization. However, the locomotion level in rats receiving saline did not show systematic changes across days (F6, 60 = 1.57, p = 0.17). CONDITIONED HYPERACTIVITY DURING
REACTIVATION SESSIONS During reactivation and intervention sessions, no data were recorded for rats that received propranolol in their home cages. Fig. 2 presented the locomotor activity
data for Mor-R-Pro, Mor-R-Sal and Sal groups. Repeated two-way ANOVA showed an effect of days (F1, 30 = 28.09; p < 0.001), an interaction of group × days (F2, 30 = 6.98; p = 0.003) but no
effect of groups (F2, 30 = 2.6; p = 0.091). On Day 10, one-way ANOVA revealed a significant group effect (F2, 30 = 3.35, p = 0.049). Post-hoc test showed that the locomotor activity of
Mor-R-Pro group was significantly higher than Sal group (p = 0.021), while there was no significant difference between Mor-R-Pro group and Mor-R-Sal group (p = 0.634). Mor-R-Sal group
exhibited higher locomotion than Sal group (p = 0.06). These results indicated that Mor-R-Pro and Mor-R-Sal groups expressed similar conditioned locomotor activity. On Day 11, one-way ANOVA
for groups reached significant level (F2, 30 = 4.17, p = 0.025). Post-hoc test showed that the locomotor activity of Mor-R-Pro group was significantly lower than Mor-R-Sal group (p = 0.009)
but there was no significant difference as compared to Sal group (p = 0.429). However, the locomotor activity of Mor-R-Sal group was significantly higher than Sal group (p = 0.05). These
results suggest that propranolol administration after reactivation can disrupt the conditioned hyperactivity 24 h later. CONDITIONED HYPERACTIVITY TEST On Day 14, all groups were tested for
conditioned locomotor activity. As shown in Fig. 3A, one-way ANOVA for the 20-min locomotor activity revealed a significant group effect (F3, 37 = 4.29, p = 0.011). The locomotor activity
level of Mor-R-Sal and Mor-NR-Pro groups were significantly higher than Sal group (p = 0.05 and p = 0.003, respectively); however, the locomotor activity level between Mor-R-Pro group and
Sal group was not significantly different (p = 0.215). As shown in Fig. 3B, one-way ANOVA for the 60-min locomotor activity after saline injection also revealed significant group effect (F3,
37 = 5.55, p = 0.003). The locomotor activity of Sal and Mor-R-Pro groups were significantly lower than Mor-R-Sal (p = 0.011, p = 0.033) and Mor-NR-Pro (p = 0.001, p = 0.005) groups. These
results indicated that propranolol administration following CS reactivation is enough to disrupt the reconsolidation for conditioned hyperactivity and this effect could not be attributed to
propranolol administration per se, for the conditioned hyperactivity of Mor-NR-Pro group remained higher conditioned hyperactivity. THE LOCOMOTOR SENSITIZATION TEST On Day 15, a morphine (3
mg/kg) challenge was used to test locomotor sensitization. As shown in Fig. 4A, one-way ANOVA for the locomotor activity revealed significant group effects (F3, 37 = 10.57, p < 0.001).
The locomotor activity of Mor-R-Pro, Mor-R-Sal and Mor-NR-Pro groups was significantly higher than that of Sal group (all p < 0.001), whereas no significant differences were found among
Mor-R-Pro, Mor-R-Sal and Mor-NR-Pro groups (p > 0.05). These results suggest that the locomotor sensitization remained unaltered in spite of the fact that the conditioned hyperactivity
was disrupted by propranolol treatment after CS reactivation. On Day 22, after a small dose of morphine (2 mg/kg) reactivation and propranolol treatment, another challenge dose of morphine
(3 mg/kg) was used to test the locomotor sensitization. As shown in Fig. 4B, one-way ANOVA revealed significant group effects (F3, 37 = 17.8, p < 0.001). The locomotor activity of
Mor-R-Pro, Mor-R-Sal and Mor-NR-Pro groups was significantly higher than that of Sal group (p < 0.001 for Mor-R-Pro and Mor-R-Sal, p = 0.047 for Mor-NR-Pro). Unexpectedly, the locomotor
activity of Mor-R-Pro group was significantly higher than Mor-R-Sal and Mor-NR-Pro group (p = 0.01 and p < 0.001). These results suggest that a drug-primed reactivation followed by
propranolol treatment could not disrupt the expression of locomotor sensitization. In contrast, propranolol enhanced the magnitude of locomotor sensitization to morphine. DISCUSSION Two
methods were used in the current study to measure the conditioned hyperactivity: one was the 20-min recording in the locomotion chambers without any treatment and the other was an additional
60-min locomotion recording, which has been used by other researchers35,36. The results of both methods showed that propranolol treatment following reactivation inhibited the development of
conditioned hyperactivity, which is consistent with previous studies which have demonstrated post-retrieval impairment effect of drug-mediated behaviors27,28,31. The disrupting effects of
propranolol could not be attributed to the drug _per se_, because the conditioned hyperactivity remained unaltered if propranolol was given in the absence of reactivation. Since the goal of
reconsolidation studies is to retrieve the original memory trace, most studies accomplish this by using CS re-exposure, which is essentially a short extinction session. For example, animals
are often put into the test chambers without the presence of drug, or they perform an instrumental behavior for a drug-associated cue, or they are presented with the cue non-contingently33.
According to trace dominance theory37, the extinction and reconsolidation processes compete with each other and the amnestic agents will block the dominance process. The prevailing concept
in reconsolidation studies is that reconsolidation sessions need to be short to avoid extinction. The time period of exposure for reactivation varies markedly depending on the type of
experiment. For example, in fear conditioning studies, reactivation is often only 30 s but in drug abuse studies the re-exposure session can be as much as 30 min because enough time is
typically allowed for the animal to perform the behavior33. The reconsolidation process was also influenced by the strength of memories. For instance, strong memories were found to be more
resistant to reconsolidation, but could be rendered labile again only if the reminder session was prolonged13,18. In the current study, 20 min re-exposure to training chamber were chosen,
which is based on our pilot study that the current time period is enough to develop conditioned hyperactivity and to avoid extinction process. To effectively disrupt the reconsolidation
process, the reactivation and amnestic intervention were carried out twice based on previous studies20,26,38. Noradrenaline neurotransmission plays a critical role in learning and memory
processes. Specifically, β-adrenoceptors are involved in long-term potentiation39 and consolidation of memory40. The β-adrenergic receptor activation is also important for post-retrieval
stabilization of memories, as systemic injections with the β-adrenoceptor antagonist propranolol impairs the expression of aversive memories in rats that received reactivation41. Using
self-administration paradigm, propranolol persistently disrupts reconsolidation of Pavlovian associations between environmental conditioned stimuli and appetitive reinforcers when
administered immediately after memory reactivation25. In addition, reactivation of drug-related memories and concomitant propranolol administration disrupt subsequent expression of
cocaine-27 and morphine-29,31 induced CPP. In this study, the conditioned hyperactivity induced by Pavlovian pairing of context and morphine could also be disrupted by administration of
propranolol after the retrieval trial, which is consistent with previous reports. In the earlier studies, many researchers used protein synthesis inhibitors to disrupt the reconsolidation
process. Although protein synthesis inhibitors can effectively attenuate _de novo_ protein synthesis, their non-specific toxicity precludes their clinical use. The β-adrenoceptor antagonists
are already available for human use and there is evidence for use of propranolol as an amnestic to treat posttraumatic stress disorder (PTSD)42,43. In this sense, propranolol which
interferes with memory reconsolidation processes might open the door to novel treatment for drug addiction and other psychiatric disorders. Although conditioned hyperactivity could be
disrupted by CS reactivation and propranolol administration, the morphine challenge results suggest that the expression of locomotor sensitization could not be disrupted by propranolol
administration following CS reactivation. Considering drug-priming is a powerful reminder of the drug-associated memory, rats were given an injection of morphine (2 mg/kg) followed by 30 min
freely activity in locomotion chamber, which served as a CS + US retrieval trial. A small dose of morphine (2 mg/kg) was chosen for the reason that we wish to reactivate the unconditioned
drug effect in the premise not to enhance sensitization effect as much as possible. The reactivation duration (30 min) was chosen for the unconditioned morphine effect reach a higher level
in 30 min. However, CS + US reactivation and propranolol intervention also could not disrupt locomotor sensitization. Both results suggest that the locomotor sensitization effect could not
be erased by disrupting the reconsolidation process. Several possibilities could attribute to the differential effects of propranolol on the reconsolidation of context-associated
hyperactivity and morphine-induced sensitization. First, in the current study, morphine-induced sensitization develops during conditioned training (Fig. 1), whereas context-associated
hyperactivity requires the longer periods and perhaps a period of abstinence. These results are similar to the study conducted by the group of Li6, although there are differences in training
session and abstinence period. Also, Kosowski et al4 have shown that the conditioned response to nicotine could not be demonstrated until the sensitized locomotor activity response reached
a plateau phase, which suggests that a maximal level in the expression of behavioural sensitization to nicotine precedes the onset of conditioned increase of locomotor activity. Therefore,
we can infer that the memory associative strength of context-associated hyperactivity is smaller than that of morphine-induced sensitization. Previous studies have shown that stronger memory
is less readily reactivated18. Second, Anagnostaras and colleagues7,44 have demonstrated that there are three memory processes underling locomotor sensitization: drug exposure causes
non-associative cellular changes which are essential for locomotor sensitization; drug exposure initiates an inhibitory associative process which attenuates the expression of locomotor
sensitization in the unpaired environmental context; drug exposure initiates an excitatory associative process which facilitates the expression of locomotor sensitization in the paired
environmental context. In their experiments, rats received repeated injections of amphetamine or saline in group-specific environments. Following these treatments some groups were given an
electroconvulsive shock when memories of the drug experience were reactivated (and therefore vulnerable to disruption) in order to produce retrograde amnesia. Their results have shown that
the electroconvulsive shock affects the expression of sensitization in unpaired animals but not in paired animals. The experiment conducted by the group of Anagnostaras7 is also considered a
reconsolidation study, which showed that electroconvulsive shock after reactivation could disrupt the inhibitory associative effect, whereas it has no effect on locomotor sensitization
caused by non-associative effect. However, their results have also indicated that the electroconvulsive shock after reactivation could not disrupt the excitatory associative effect, which is
in contrast to our results. We have confirmed that the association between drug and context can be disrupted by blocking the reconsolidation process, whereas the non-association of
neuroplasticity induced by intermittent drug treatment is not simultaneously disrupted by the same intervention. These results imply a dissociated memory mechanism between conditioned
hyperactivity and locomotor sensitization. Third, the behavioral activating effects of addictive drugs appear to be mediated by their actions on mesotelencephalic and related circuitry,
especially dopaminergic projections to the striatum originating from the ventral tegmental area and substantia nigra and glutamate projections originating from the neocortex45,46,47,48,49.
Moreover, there are corresponding persistent presynaptic and postsynaptic changes in monoamine and glutamate neurotransmission in the striatum of sensitized animals8,47,50, which may be
related to persistent changes in the morphology of neurons in the nucleus accumbens and prefrontal cortex51,52. McDonald and White53 postulated a triple memory system which included the
hippocampal formation, the amygdala and the dorsal striatum. Within this account, the hippocampus is held to be responsible for the acquisition and retrieval of declarative memory and
stimulus-stimulus associations. The amygdala system is believed to mediate Pavlovian associations between stimuli and contingencies, both reinforcing and aversive. The dorsal striatum
mediates implicit, dopamine-modulated habit-based learning. Based on this theory, the neural substrate of conditioned hyperactivity is thought to be involved in the amygdala system, whereas
locomotor sensitization appears to be mediated by mesotelencephalic dopamine system. The separate neural substrates may contribute to the differential effects of propranolol on conditioned
hyperactivity and locomotor sensitization. Bernardi et al32 have noted that rats given anisomycin immediately after a reactivation session show decreased activity as compared to the saline
group in response to a low-dose of cocaine challenge. Carrera et al54 have also shown that a single post-conditioning trial treatment with a low dose of apomorphine could reverse
apomorphine-induced locomotor sensitization in paired group, using a conventional paired/unpaired Pavlovian protocol. However, these reports are different from the current study in two
important aspects: first, those studies did not distinguish the associative and non-associative effects in the challenge test and thus it is not clear which component experiences memory
reconsolidation; second, the conditioning session usually consists of multiple drug-context pairing in a typical locomotor sensitization paradigm and one-shot procedure induced sensitization
used by previous studies might have different sensitization magnitude from the typical intermittent administration procedure. According to the current results, it is suggested that
propranolol could not interact with the reconsolidation process of locomotor sensitization, which still needs to be confirmed. It should be admitted that there were already differences in
the response to the conditioned context in morphine-treated groups after previous propranolol/context exposure on days 10–11, so when they were re-exposed to the context on days 18–19, the
precise associations reactivated by this context/morphine exposure might be different, which precluded drug associated memory to go into labile state. This may be another reason for the
failure of propranolol to disrupt morphine sensitization. Unexpectedly, although a drug-priming reactivation and propranolol treatment did not disrupt the locomotor sensitization, a small
dose of morphine injection and propranolol intervention enhanced the locomotor sensitization effect. It appears that a delayed interaction between propranolol and morphine enhanced the
locomotor sensitization effect of morphine. Within the mesocorticolimbic dopamine system, several interactions between dopamine and noradrenaline transmission have been described that may
underlie the effect of propranolol on the psychomotor effect of psychostimulant. Harris et al55 reported that dopamine levels in the accumbens increased by an average of 700% over baseline
levels in the presence of combined cocaine and propranolol. Vanderschuren et al56 have found that propranolol enhanced the psychomotor stimulant effect of amphetamine and cocaine. However,
the present study shows that propranolol is critical for the long term sensitizing effects of morphine. Because of the limited literature, it remains unclear by which mechanism propranolol
interacts with morphine to enhance the sensitizing effects of morphine. In conclusion, this study indicates that the conditioned hyperactivity caused by environmental cues associated with
morphine treatment or by injection of saline can be erased by administration of propranolol after retrieval of related memory, which lasts for a much longer time. However, the
morphine's sensitization effects induced by intermittent morphine administration cannot be blocked by disrupting the reconsolidation process. The results of the study also support
propranolol as a useful pharmacological tool for blocking reconsolidation of drug-associated memories. METHODS All experimental procedures were carried out in accordance with the 1996
National Institutes of Health Guide for the Care and Use of Laboratory Animals and the experimental procedures were approved by the Local Committee of Animal Use and Protection. ANIMALS
Adult male Wistar rats (200–220 g, Academy of Military Medical Science Animal Center, Beijing, China) were housed in standard lab Plexiglas cages (45 × 30 × 25 cm, length × width × height, 3
rats/cage) in a weather-controlled ventilated colony room on a 12-h-light/12-h-dark cycle (experiments were conducted during the light period) with free access to water and food in the home
cages. DRUGS Morphine hydrochloride (Shenyang First Pharmaceutical Factory, Shenyang, China) and propranolol (Sigma-Aldrich, St. Louis, MO) were dissolved with saline and injected
intraperitoneally (i.p.) at a volume of 1 ml/kg. APPARATUS Locomotor activity was measured by an automated video tracking system with four customer-made activity chambers as described
previously (Li et al., 2010). The chambers were made of black Perspex plastics (40 × 40 × 50 cm, length × width × height). A video camera was mounted at the top of the chamber, which was
connected to a PC to record the locomotion of rats. The video documents (stored in the computer) were analyzed by the LA analysis software (Institute of Psychology, Chinese Academy of
Sciences, Beijing, China). The locomotor activity was expressed as the total distance traveled for a predetermined period of time. To increase the salience of the CS, as described in Li et
al. (2010), 1.5 ml of 50% acetic acid dropped on absorbent cotton served as the CS and was replaced daily immediately before the session started. The cotton was held in a porous metal
container and put in the top corner of the chamber out of the reach of rats. BEHAVIORAL EXPERIMENTS The acquisition of conditioned hyperactivity and behavioral sensitization sessions has
been described in detail previously6 with modifications. Briefly, 41 rats were randomly assigned into one of four groups (see Table 1). These sessions were conducted during seven consecutive
days (Day 1–7). On each conditioning day, all rats were put into the locomotion chambers for 20 min (paired with CS) with an injection of morphine (5 mg/kg) or saline afterwards. The
locomotor activity was measured for the following 120 min. All groups were maintained in their home cages without any drug treatment during Day 8–9. The dose of morphine used in this study
(5 mg/kg) was chosen based upon a previous report6 and our pilot studies (data not shown) which revealed that this dose of morphine produced the most robust hyperactivity and locomotor
sensitization. Two days after the conditioning sessions, rats were transported into the locomotion chambers for a 20-min re-exposure trial on Day 10. This re-exposure manipulation served as
a CS retrieval trial intended to reactivate the association between morphine and CS in rats given morphine during training sessions. Immediately after re-exposure, rats were administered
with propranolol (10 mg/kg) or saline and placed back into their home cages. The non-reactivation control group received propranolol in home cages. The dose of propranolol used in this study
(10 mg/kg) was chosen based upon previous reports25,31. In order to effectively disrupt the reconsolidation process, the CS re-exposure trial and amnestic intervention were repeated on Day
11. All groups were remained undisturbed in their home cages during Day 12–13. On Day 14, the conditioned hyperactivity of rats was first tested for 20 min in locomotion chambers. To fully
model the CS, rats were then given an injection of saline and their locomotion was recorded for another 60-min in a drug-free state35,36. On Day 15, all rats received an injection of
morphine (3 mg/kg) and the locomotor activity was measured for 120 min to serve as locomotor sensitization test. Two days after locomotor sensitization test, on Day 18, rats were transported
into the locomotor cages for 30 min following an injection of morphine (2 mg/kg, i.p.), which served as a CS + US retrieval trial. Immediately after re-exposure, rats were administered
propranolol (10 mg/kg) or saline and placed back into home cages. The CS + US re-exposure trial and amnestic intervention were repeated on Day 19. The non-reactivation control group received
propranolol in home cages. All groups were remained undisturbed in their home cages during Days 20–21. On Day 22, all rats were given a second morphine (3 mg/kg) challenge test. DATA
ANALYSES Two-way mixed factorial ANOVAs were performed on the data with the between-subjects factors of group and within-subjects factors of day for the conditioning and
reactivation/intervention sessions. When a significant effect of group versus day interaction was recorded, one-way ANOVA was used to analyze the differences in the conditioned hyperactivity
and locomotor sensitization among different groups. Post hoc analyses (bonferroni test) were performed for assessing specific group comparison wherever indicated by ANOVA results (with p
< 0.05). The behavioral data obtained from the conditioning test and sensitization test were analyzed using a one-way ANOVA. Wherever indicated by the ANOVA results (with p < 0.05),
possible differences among groups were analyzed by bonferroni test. The data were expressed as means ± SEM. The levels of statistical significance were set at p < 0.05. REFERENCES *
Aston-Jones, G. & Harris, G. C. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology 47, 167–179 (2004). Article CAS PubMed Google Scholar *
Foltin, R. W. & Haney, M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl.) 149, 24–33 (2000). Article CAS Google Scholar *
Lee, J. L., Milton, A. L. & Everitt, B. J. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J. Neurosci. 26, 5881–5887 (2006). Article
CAS PubMed PubMed Central Google Scholar * Kosowski, A. R. & Liljequist, S. Behavioural sensitization to nicotine precedes the onset of nicotine-conditioned locomotor stimulation.
Behav. Brain Res. 156, 11–17 (2005). Article CAS PubMed Google Scholar * Hotsenpiller, G. & Wolf, M. E. Conditioned Locomotion Is Not Correlated with Behavioral Sensitization to
Cocaine: An Intra-Laboratory Multi-Sample Analysis. Neuropsychopharmacology 27, 924–929 (2002). Article CAS PubMed Google Scholar * Li, X., Li, J., Zhu, X., Cui, R. & Jiao, J.
Effects of physostigmine on the conditioned hyperactivity and locomotor sensitization to morphine in rats. Behav. Brain Res. 206, 223–228 (2010). Article CAS PubMed Google Scholar *
Anagnostaras, S. G., Schallert, T. & Robinson, T. E. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology 26, 703–715 (2002). Article CAS
PubMed Google Scholar * Robinson, T. E. & Berridge, K. C. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95, S91–S117 (2000). PubMed Google
Scholar * Robinson, T. E. & Berridge, K. C. Addiction. Annu. Rev. Psychol. 54, 25–53 (2003). Article PubMed Google Scholar * Robinson, T. E. & Berridge, K. C. The incentive
sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 3137–3146 (2008). Article PubMed PubMed Central Google Scholar * Nader, K. Memory
traces unbound. Trends Neurosci. 26, 65–72 (2003). Article CAS PubMed Google Scholar * Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the
amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). Article ADS CAS PubMed Google Scholar * Tronson, N. C. & Taylor, J. R. Molecular mechanisms of memory
reconsolidation. Nat. Rev. Neurosci. 8, 262–275 (2007). Article CAS PubMed Google Scholar * Torras-Garcia, M., Lelong, J., Tronel, S. & Sara, S. J. Reconsolidation after remembering
an odor-reward association requires NMDA receptors. Learn. Mem. 12, 18–22 (2005). Article PubMed PubMed Central Google Scholar * Dębiec, J. & LeDoux, J. E. Disruption of
reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–272 (2004). Article CAS PubMed Google Scholar * Kida,
S. et al. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 5, 348–355 (2002). Article CAS PubMed Google Scholar * Lee, J. L. C., Everitt, B. J. &
Thomas, K. L. Independent Cellular Processes for Hippocampal Memory Consolidation and Reconsolidation. Science 304, 839–843 (2004). Article ADS CAS PubMed Google Scholar * Suzuki, A.
et al. Memory Reconsolidation and Extinction Have Distinct Temporal and Biochemical Signatures. J. Neurosci. 24, 4787–4795 (2004). Article CAS PubMed PubMed Central Google Scholar *
Tronson, N. C., Wiseman, S. L., Olausson, P. & Taylor, J. R. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 9,
167–169 (2006). Article CAS PubMed Google Scholar * Brown, T. E. et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place
preference. Learn. Mem. 14, 214–223 (2007). Article CAS PubMed PubMed Central Google Scholar * Kelley, J. B., Anderson, K. L. & Itzhak, Y. Long-term memory of cocaine-associated
context: disruption and reinstatement. Neuroreport 18, 777–780 (2007). Article CAS PubMed Google Scholar * Milekic, M. H., Brown, S. D., Castellini, C. & Alberini, C. M. Persistent
disruption of an established morphine conditioned place preference. J. Neurosci. 26, 3010–3020 (2006). Article CAS PubMed PubMed Central Google Scholar * Miller, C. A. & Marshall,
J. F. Molecular Substrates for Retrieval and Reconsolidation of Cocaine-Associated Contextual Memory. Neuron 47, 873–884 (2005). Article CAS PubMed Google Scholar * Valjent, E.,
Corbille, A. G., Bertran-Gonzalez, J., Herve, D. & Girault, J. A. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place
preference. Proc. Natl. Acad. Sci. U.S.A. 103, 2932–2937 (2006). Article ADS CAS PubMed PubMed Central Google Scholar * Milton, A. L., Lee, J. L. C. & Everitt, B. J.
Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn. Mem. 15, 88–92 (2008). Article PubMed Google Scholar * Lee,
J. L. C., Di Ciano, P., Thomas, K. L. & Everitt, B. J. Disrupting Reconsolidation of Drug Memories Reduces Cocaine-Seeking Behavior. Neuron 47, 795–801 (2005). Article CAS PubMed
Google Scholar * Bernardi, R. E., Lattal, K. M. & Berger, S. P. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport 17, 1443–1447 (2006). Article CAS
PubMed Google Scholar * Fricks-Gleason, A. N. & Marshall, J. F. Post-retrieval β-adrenergic receptor blockade: Effects on extinction and reconsolidation of cocaine-cue memories.
Learn. Mem. 15, 643–648 (2008). Article PubMed PubMed Central Google Scholar * Robinson, M. J. F., Ross, E. C. & Franklin, K. B. J. The effect of propranolol dose and novelty of the
reactivation procedure on the reconsolidation of a morphine place preference. Behav. Brain Res. 216, 281–284 (2011). Article CAS PubMed Google Scholar * Robinson, M. J. F. &
Franklin, K. B. J. Reconsolidation of a morphine place preference: Impact of the strength and age of memory on disruption by propranolol and midazolam. Behav. Brain Res. 213, 201–207 (2010).
Article CAS PubMed Google Scholar * Robinson, M. J. F. & Franklin, K. B. J. Central but not peripheral β-adrenergic antagonism blocks reconsolidation for a morphine place
preference. Behav. Brain Res. 182, 129–134 (2007). Article CAS PubMed Google Scholar * Bernardi, R. E., Lattal, K. M. & Berger, S. P. Anisomycin Disrupts a Contextual Memory
Following Reactivation in a Cocaine-Induced Locomotor Activity Paradigm. Behav. Neurosci. 121, 156–163 (2007). Article CAS PubMed Google Scholar * Sorg, B. A. Reconsolidation of drug
memories. Neurosci. Biobehav. Rev. 36, 1400–1417 (2012). Article PubMed PubMed Central Google Scholar * Miller, C. A. & Sweatt, J. D. Amnesia or retrieval deficit? Implications of a
molecular approach to the question of reconsolidation. Learn. Mem. 13, 498–505 (2008). Article CAS Google Scholar * Bevins, R. A., Besheer, J. & Pickett, K. S. Nicotine-conditioned
locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol. Biochem. Behav. 68, 135–145 (2001). Article CAS PubMed Google Scholar * Reid, M.
S., Ho, L. B. & Berger, S. P. Behavioral and neurochemical components of nicotine sensitization following 15-day pretreatment: studies on contextual conditioning. Behav. Pharmacol. 9,
137–148 (1998). CAS PubMed Google Scholar * Eisenberg, M., Kobilo, T., Berman, D. E. & Dudai, Y. Stability of Retrieved Memory: Inverse Correlation with Trace Dominance. Science 301,
1102–1104 (2003). Article ADS CAS PubMed Google Scholar * Fan, H. et al. Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav. Brain
Res. 208, 522–527 (2010). Article CAS PubMed Google Scholar * Gelinas, J. N. & Nguyen, P. V. β-Adrenergic Receptor Activation Facilitates Induction of a Protein Synthesis-Dependent
Late Phase of Long-Term Potentiation. J. Neurosci. 25, 3294–3303 (2005). Article CAS PubMed PubMed Central Google Scholar * McGaugh, J. L. The amygdala modulates the consolidation of
memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28 (2004). Article CAS PubMed Google Scholar * Przybyslawski, J., Roullet, P. & Sara, S. J. Attenuation of
emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J. Neurosci. 19, 6623–6628 (1999). Article CAS PubMed PubMed Central Google Scholar *
Vaiva, G. et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol. Psychiat. 54, 947–949 (2003). Article CAS PubMed Google
Scholar * Schwabe, L., Nader, K., Wolf, O. T., Beaudry, T. & Pruessner, J. C. Neural Signature of Reconsolidation Impairments by Propranolol in Humans. Biol. Psychiat. 71, 380–386
(2012). Article CAS PubMed Google Scholar * Anagnostaras, S. G. & Robinson, T. E. Sensitization to the Psychomotor Stimulant Effects of Amphetamine: Modulation by Associative
Learning. Behav. Neurosci. 110, 1397–1414 (1996). Article CAS PubMed Google Scholar * Carlezon, W. A. & Wise, R. A. Rewarding actions of phencyclidine and related drugs in nucleus
accumbens shell and frontal cortex. J. Neurosci. 16, 3112–3122 (1996). Article CAS PubMed PubMed Central Google Scholar * Koob, G. F. & Bloom, F. E. Cellular and molecular
mechanisms of drug dependence. Science (1988). * Vanderschuren, L. J. & Kalivas, P. W. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of
behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl.) 151, 99–120 (2000). Article CAS Google Scholar * Wise, R. A. & Bozarth, M. A. A
psychomotor stimulant theory of addiction. Psychol. Rev. 94, 469 (1987). Article CAS PubMed Google Scholar * Wolf, M. E. The role of excitatory amino acids in behavioral sensitization to
psychomotor stimulants. Prog. Neurobiol. 54, 679–720 (1998). Article CAS PubMed Google Scholar * Robinson, T. E. & Becker, J. B. Enduring changes in brain and behavior produced by
chronic amphetamine administration: a review and evaluation of animalmodels of amphetamine psychosis. Brain Res. 396, 157–198 (1986). Article CAS PubMed Google Scholar * Robinson, T. E.
& Kolb, B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 17, 8491–8497 (1997).
Article CAS PubMed PubMed Central Google Scholar * Robinson, T. E. & Kolb, B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal
cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 11, 1598–1604 (1999). Article CAS PubMed Google Scholar * McDonald, R. J. & White, N. M. A triple
dissociation ofmemory systems: hippocampus, amygdala and dorsal striatum. Behav. Neurosci. 107, 3–22 (1993). Article CAS PubMed Google Scholar * Carrera, M. P., Carey, R. J., Dias, F. R.
C. & de Matos, L. W. Reversal of apomorphine locomotor sensitization by a single post-conditioning trial treatment with a low autoreceptor dose of apomorphine: a memory re-consolidation
approach. Pharmacol. Biochem. Behav. 99, 29–34 (2011). Article CAS PubMed Google Scholar * Harris, G. C., Hedaya, M. A., Pan, W. J. & Kalivas, P. β-adrenergic antagonism alters the
behavioral and neurochemical responses to cocaine. Neuropsychopharmacology 14, 195–204 (1996). Article CAS PubMed Google Scholar * Vanderschuren, L. J., Beemster, P. & Schoffelmeer,
A. N. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl.) 169, 176–185 (2003). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS This study was supported by a grant (10YJAXLX10) from the China social science university humanity program of Ministry of Education of People's Republic of
China and a grant (2013-GH-433) from Humanities and Social Science Fund of He'nan educational Commission of China. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Psychology, Beijing Key Laboratory of Learning and Cognition, Capital Normal University, 100048, Beijing, China Shuguang Wei & Xinwang Li * College of Education Science and Teacher
Development, Henan Normal University, Xinxiang, 453007, China Shuguang Wei Authors * Shuguang Wei View author publications You can also search for this author inPubMed Google Scholar *
Xinwang Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.W. and X.L. designed and performed the research as well as wrote the paper.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Wei, S., Li, X. Differential effects of propranolol on conditioned hyperactivity and locomotor sensitization induced by morphine in rats. _Sci Rep_ 4, 3786 (2014).
https://doi.org/10.1038/srep03786 Download citation * Received: 29 November 2013 * Accepted: 02 January 2014 * Published: 21 January 2014 * DOI: https://doi.org/10.1038/srep03786 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
