Novel antarctic yeast adapts to cold by switching energy metabolism and increasing small rna synthesis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The novel extremophilic yeast _Rhodotorula frigidialcoholis_, formerly _R_. JG1b, was isolated from ice-cemented permafrost in University Valley (Antarctic), one of coldest and
driest environments on Earth. Phenotypic and phylogenetic analyses classified _R. frigidialcoholis_ as a novel species. To characterize its cold-adaptive strategies, we performed mRNA and
sRNA transcriptomic analyses, phenotypic profiling, and assessed ethanol production at 0 and 23 °C. Downregulation of the ETC and citrate cycle genes, overexpression of fermentation and
pentose phosphate pathways genes, growth without reduction of tetrazolium dye, and our discovery of ethanol production at 0 °C indicate that _R. frigidialcoholis_ induces a metabolic switch
from respiration to ethanol fermentation as adaptation in Antarctic permafrost. This is the first report of microbial ethanol fermentation utilized as the major energy pathway in response to
cold and the coldest temperature reported for natural ethanol production. _R_. _frigidialcoholis_ increased its diversity and abundance of sRNAs when grown at 0 versus 23 °C. This was
consistent with increase in transcription of Dicer, a key protein for sRNA processing. Our results strongly imply that post-transcriptional regulation of gene expression and mRNA silencing
may be a novel evolutionary fungal adaptation in the cryosphere. SIMILAR CONTENT BEING VIEWED BY OTHERS MORPHOLOGICAL AND PHYSIOLOGICAL ADAPTATIONS OF PSYCHROPHILIC _PSEUDARTHROBACTER
PSYCHROTOLERANS_ YJ56 UNDER TEMPERATURE STRESS Article Open access 11 September 2023 A COLD SHOCK PROTEIN PROMOTES HIGH-TEMPERATURE MICROBIAL GROWTH THROUGH BINDING TO DIVERSE RNA SPECIES
Article Open access 16 March 2021 _SUBTERCOLA ENDOPHYTICUS_ SP. NOV., A COLD-ADAPTED BACTERIUM ISOLATED FROM _ABIES KOREANA_ Article Open access 15 July 2022 INTRODUCTION The majority of the
Earth’s biosphere exists at permanently cold temperatures, below 5 °C, and includes multiple cryoenvironments (<0 °C), many of which are characterized by some of the most dry, low
biomass, cold, and salty conditions on Earth. Despite these harsh conditions, microorganisms capable of metabolic activity and even growth in situ have been reported in these habitats and
include bacteria, archaea, algae, fungi [1,2,3]. These cold-adapted microorganisms are termed as psychrophilic (optimum temperature below 15 °C) or psychrotolerant (optimum above 15 °C) [4],
and are able to maintain viability for thousands of years in glacial ice [5, 6]. Microbial communities in these environments have to overcome numerous biochemical and physiological
challenges, including low water and nutrient availability, high oxidative stress, high solar irradiation, and multiple freeze-thaw cycles, coupled with a major decrease in membrane fluidity,
enzymatic activity, and protein folding, and with the creation of stable secondary inhibitory DNA/RNA structures [4, 7,8,9]. While numerous studies have investigated bacteria, archaea and
algae from Arctic and Antarctica environments [4, 10, 11], there has been a recent interest in fungi inhabiting these environments [12,13,14]. Many fungal species have been isolated and
characterized from a diversity of extreme environments, such as brines [15], Arctic glaciers, and Antarctica rocks and deserts [5, 16, 17]. Fungi play key roles in the cryosphere as they are
important facilitators of primary biomass production through endophytic and lichenic relationships [18, 19] and are involved in the nutrients recycling [20]. Although many basidiomycetous
yeasts are adapted to low temperatures and detected in a broad range of cold ecosystems [21, 22], their adaptation strategies to low temperatures are not fully understood. Molecular
mechanisms enabling yeast survival include the production of antifreeze and cold-active proteins, compatible solutes, and an increase in membrane fluidity [5, 23]. For example, the
heterotrophic _Rhodotorula_ yeasts are unicellular and pink-pigmented [24, 25] and have been isolated in many cold habitats [26]; numerous cold-adapted species, including _R. aurantiaca_,
_R. psychrophila_, _R. psychrophenolica_, _R. glacialis_, and _R. himalayensis_ [27, 28] have been identified. More recently, we isolated the putative novel psychrotolerant _Rhodotorula_
JG1b strain from ~150,000-year-old ice-cemented permafrost soil from University Valley, in the McMurdo Dry Valleys of Antarctica [24], one of the coldest and driest places on Earth [29].
_Rhodotorula_ JG1b was one of only six microorganisms isolated and sequenced form this ice-cemented permafrost. It was capable of growth at temperatures as low as −10 °C [1] and tolerated up
to 15% NaCl and 12% perchlorate [24]. In this study, _Rhodotorula_ JG1 was used as a model yeast to determine its adaptations to cold temperatures and how it survives in one of the coldest
environments on Earth. Our objectives were to determine the phylogenetic position of _Rhodotorula_ JG1b within the _Rhodotorula_ genus and to identify and characterize its metabolic activity
pathways and regulatory mechanisms in response to cold. To determine _R. frigidialcoholis’_ response to colder temperatures, we characterized this strain’s metabolic capabilities at 0 °C,
performed transcriptomic mRNAseq analysis in cultures grown at 0 and 23 °C, and assessed this species’ ethanol production capability at 0 °C compared to 23 °C. In addition, we analyzed the
short non-coding RNAs to determine their role in post-transcriptional gene regulation to cold adaptation. MATERIALS AND METHODS TAXONOMIC ANALYSIS _Rhodotorula_ JG1b was isolated from
ice-cemented permafrost soil in University Valley (Antarctica) (24). To determine the phylogenetic position of _Rhodotorula_ JG1b, we performed phylogenetic, phenotypic, metabolic, and
physiological characterization of this strain. Phylogenetic characterization and analyses were performed using the small and large subunits of the rRNA gene, the internal transcribed spacers
(ITS) 1 and 2, translation elongation factor 1-α (TEF), and cytochrome b as well as publicly available whole genome sequences of the _Rhodotorula_ genus (Supplementary Fig. S1). Detailed
methodology of the phylogenetic analyses is described in the Supplementary material (Supplementary File 1 and Supplementary Fig. S2). The morphology of the strain was determined by growth on
potato dextrose agar (PDA), malt extract agar (MEA), cornmeal agar (CMA), and yeast extract peptone dextrose (YPD) [30], after incubation at 15 °C for 14 days. Morphological characters of
pure cultures were observed on MEA with Nomarski interference contrast optics on an Olympus BX-51 microscope, colony color was described according to [31]. Growth at different temperatures
(0, 4, 15, 20, 24, 30 and 37 °C) was determined in microtiter plates in liquid medium yeast nitrogen base. Three inocula were used (A, B, C) of optical density 0.4, 0.2, and 0.02,
respectively, with 8 replicates/inocula. The growth curves were determined via OD at 590 nm. Assimilation of carbon and nitrogen sources were studied on three different sets of Phenotypic
MicroArray (PM; Biolog) plates, PM1 MicroPlate and PM2A MicroPlate for carbon metabolism, and PM3B MicroPlate for nitrogen metabolism, as described by Viti et al., incubated at 20 °C for up
to 14 days. Fermentation of D-glucose was tested in liquid medium with a 2% solution of sugar with Bromothymol blue and Durham tube [30], after an incubation of 12 days at 5, 15, 20, 30, and
37 °C. CULTURING AND GROWTH CONDITIONS For all subsequent experiments, _Rhodotorula_ JG1b was cultured on PDB agar (HIMEDIA, Shenzhen, China). Biological triplicates of _Rhodotorula_ JG1b
were grown in 50 ml liquid cultures to early exponential phase in PDB at 0 °C without shaking, and at 23 °C with shaking at 150 RPM. Growth was monitored using OD600 and converted to viable
cell numbers/ml based on CFU counts. Lack of shaking was necessary for the 0 °C treatment to allow growth at this low temperature since growth was not observed under shaking conditions at 0
°C. We separately ensured that the 0 °C non-shaking cultures remained aerobic (11.5 ± 0.98 mg O2/l) during growth by monitoring O2 concentrations using a Piccolo2 Fiber-Optic Oxygen Meter
(Pyroscience, Aachen, Germany). O2 was not limited in this treatment, due to small culturing volume, slower growth rate at low temperature, and increase of oxygen solubility at lower
temperature [32]. PHENOTYPIC MICROARRAY ANALYSIS To physiologically characterize _Rhodotorula_ JG1b metabolic capabilities under cold conditions, its assimilation of different carbon and
nitrogen sources at 0 °C was assessed using the Biolog Phenotypic MicroArray (PM) technology (Biolog, Hayward, CA, United States). PM1 MicroPlate and PM2A MicroPlate were used for
determining carbon utilization, and PM3B MicroPlate was used for nitrogen utilization. The plates were inoculated following Viti et al. protocol [33] with the following modifications: the
initial inoculum was adjusted to OD600 0.200, and the Biolog Dye Mix G was used. The duplicate plates were incubated at 0 °C, and at room temperature of 23 °C for comparison. Absorbance
readings (590 nm) were taken at days 0, 1, 2, 6, 9, 14, 21, 28, 42, 56, 70, and 91 of incubation on a SpectraMax M2e microplate reader (Molecular Devices, San Jose, CA, United States). The
measurements were normalized by the subtraction of both the _T_0 and the negative control. Absorbance values >0.2 were considered as positive values as previously described [34]. RNA
EXTRACTION, LIBRARY PREPARATION, AND SEQUENCING To perform transcriptomic analysis, biological triplicate _Rhodotorula_ JG1b cultures of each condition were grown as described in section
“Culturing and growth conditions” to early exponential phase: OD600 0.95 at 0 °C; and OD600 6.20 at 23 °C. RNA was extracted using the Direct-zol RNA Miniprep Plus kit (#R2070) from Zymo
Research (Irvine, CA, United States) on cultures with an initial resuspended pellet of approximately 108 cells following the manufacturer’s instructions. An additional step of bead beating
was also performed where resuspended cell suspensions were bead beat with 1:3 sterile glass beads (0.1 mm) for 45 s using the Mini-BeadBeater-24 (Biospec Products, Bartlesville, OK, United
States) and centrifuged at 10,000 RCF for 2 min. Following a DNAse treatment with the TURBO DNAse kit (Invitrogen, Carlsbad, CA, United States), the RNA concentration was assessed with the
Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, United States). RNA integrity was analyzed with the RNA Nano 6000 Assay Kit on the 2100 Bioanalyzer system (Agilent
Technologies, Santa Clara, CA, United States). mRNA libraries were prepared following the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, United States) with an input
material of 1.2 µg of total RNA. Libraries band sizes were confirmed on a 4% agarose gel. We prepared small RNA (sRNA) (>200 nt) libraries for sequencing since we noted higher prevalence
of sRNAs in the 0 °C cultures compared to the 23 °C cultures (Supplementary Fig. S3). sRNA libraries were prepared following the NEBNext Multiplex Small RNALibrary Prep Workflow (New England
Biolabs, Ipswich, MA, United States). The cDNA constructs were purified with the Monarch PCR and DNA kit from New England Biolabs. Libraries band sizes were assessed with the Agilent High
Sensitivity DNA kit on the 2100 Bioanalyzer system. Library bands from 105 to 210 bp were selected using the Pippin Prep 3% Agarose Gel Cassette from Sage Science (Beverly, MA, United
States). mRNA sequencing was performed by RNASeq on the MiSeq system (Illumina) using the Reagent Kit v2 50 cycles and a 1 × 55 bp single-end configuration. sRNA sequencing was performed by
RNASeq on the MiSeq system (Illumina) using the Reagent Kit v2 300 cycles and a 2 × 151 bp paired-end configuration. mRNA and sRNA raw sequence files (fastq) were uploaded to GenBank with
accession id: PRJNA631292 BIOINFORMATICS The mRNA raw sequencing reads were quasi-mapped and quantified with Salmon software [35] to the genome of _Rhodotorula_ JG1b with the GenBank number
GCA_001541205.1, and JGI IMG Genome ID 276120717 [24]. The analysis of the resulting quantifications was performed in R using the package DESeq2 [36]. Low count genes with less than 10 reads
were removed before normalization of the samples. Hierarchical clustering and principal component analyses were used to check if the samples within the treatment (biological replicates)
were more similar than between the treatments. The _p_ value threshold for differential expression was 0.05. Log2 fold-change threshold was not used, but log2 fold-change shrinking was used
for data visualization and gene ranking. Genes with a log2 fold-change (log2FC) ratio ≥∣1.5∣, and a _p_ value <0.05 were considered as differentially expressed between the two growth
conditions. Predicted protein KEGG assignments for each differentially expressed gene were downloaded from BlastKOALA website [37], to obtain function and metabolic pathway assignments.
Heatmaps of the relative abundance of transcript expression of specific pathways were created. For those heatmaps, relative gene abundances of each sample were calculated by taking gene
abundance (corrected on sequencing depth and length) and subtracting the average abundance across all samples. sRNA raw sequence data were trimmed for adapter sequences and quantified using
the CLC Genomics Workbench version 12 (https://www.qiagenbioinformatics.com/). The short rRNA fragments (rRFs)/microRNAs (miRNAs) sequences (15–30 nt) were extracted and counted from the
total sRNA sequences using the CLC Genomics Workbench Small RNA Analysis tool. Identical short rRFs/miRNAs were grouped together and low count sequences with less than 50 reads were removed.
The short rRFs/miRNAs were normalized based on sequencing coverage and to the ratio of sRNA/Total RNA, and differential expression was analyzed, with a _p_ value threshold of 0.05. PCoA
analysis (Bray–Curtis distance) was performed with the CLC Genomics Workbench version 12. The genomic origin of the ten highest expressed short rRFs/miRNAs were predicted with BLAST [38] by
comparing to _Rhodotorula_ genomes, and their potential mRNA targets were predicted using the RNAhybrid algorithm with the short rRFs/miRNAs sequences and all possible transcribed mRNA
sequences as potential targets [39, 40]. The predicted protein mRNA targets of the short rRFs/miRNAs were identified with KEGG [37]. ETHANOL PRODUCTION ASSAY To corroborate the phenotypic
and transcriptomic results, _Rhodotorula frigidialcoholis’_ ethanol production capability was assessed. Triplicate _Rhodotorula_ JG1b cultures were grown in identical media and conditions as
with transcriptomic analysis, detailed in section “Culturing and growth conditions.” OD600 of the cultures was measured at regular time points for 83 days at 0 °C and for 17 h at 23 °C.
Aliquots (1 ml) of the cultures at each time point were analyzed for ethanol production using the EnzyChrom Ethanol Assay Kit (BioAssay System, Hayward, CA, United States). Analysis of the
ethanol production was done by comparing the trendline of ethanol concentration to the trendline of cell density over time. Ethanol concentrations were normalized to the ethanol evaporation
rate that was experimentally determined using sterile PDB broth inoculated with 0.05% of ethanol. RESULTS AND DISCUSSION TAXONOMY AND DESCRIPTION OF _RHODOTORULA FRIGIDIALCOHOLIS_ SP. NOV
The _Rhodotorula_ yeast strain JG1b isolated from the upper-elevation McMurdo Dry Valleys of Antarctica [24] was determined to be a novel species based on phylogenetic and phenotypic
analyses. Bayesian analysis of aligned, concatenated 18S, ITS, D1/D2 domains of 28S rRNA, and TEF sequences of species within the genus _Rhodotorula_ resulted in a consensus tree shown in
Supplementary Fig. S1A, while the phylogenetic tree based on available complete _Rhodotorula_ genomes is shown in Supplementary Fig. S1B. According to these analyses, the JG1b strain forms a
strongly supported monophyletic group within _Rhodotorula_ species and is highly supported as the sister clade of _R. mucilaginosa_ (bootstrap = 100%). Clustering analysis of the data
obtained from 190 carbon and 95 nitrogen assimilation assays confirmed that _Rhodotorula_ JG1b is distinct from other _Rhodotorula_ species included in the Biolog phenotyping experiment
(Supplementary Fig. S1C). Contrary to _R. mucilaginosa_ and _R. alborubescens_, _Rhodotorula_ JG1b is able to assimilate L-rhamnose, dulcitol, L-sorbose, and is unable to assimilate citrate
and D-cellobiose (Supplementary Table S1). Based on the results presented here, we strongly suggest that _Rhodotorula_ sp. JG1b is a novel species in the _Rhodotorula_ genus. Accordingly,
the species is taxonomically introduced herewith as _Rhodotorula frigidialcoholis_ D. Touchette & P. Zalar, sp. nov. (frigidus in Latin meaning cold, alcohilis in Latin genitive case of
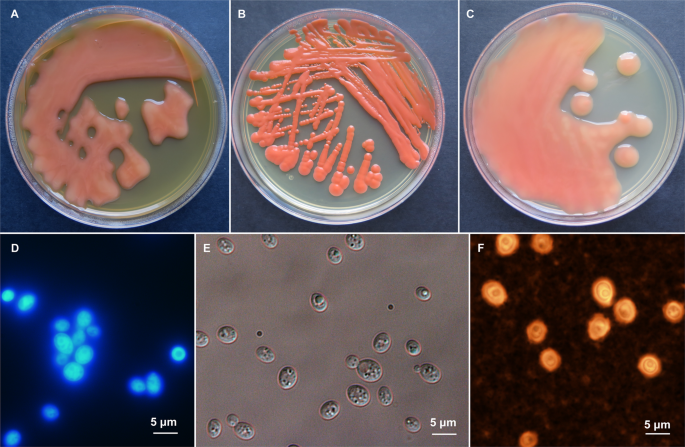
alcohol; of cold alcohol, referring to ability of alcohol production in cold). Morphological characters of _R. frigidialcoholis_ are shown in Fig. 1. Holotype for _Rhodotorula
frigidialcoholis_designated herewith: EXF-10854H, permanently preserved and metabolically inactive deposit of the Culture Collection of Extremophilic Fungi Ex (EXF), University of Ljubljana,
Slovenia. Ex-type cultures: EXF-10854 and CBS 16468 (Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands). The strain EXF-10854 was isolated as JG1b from ~150,000-year-old
ice-cemented permafrost of University Valley, the upper-elevation McMurdo Dry Valleys of Antarctica in 2009 by Jacqueline Goordial (24). DNA sequence accession numbers derived from type:
MT569975 (18S rRNA), MT569976 (D1/D2 of 28S rRNA), MT560678 (ITS), MT584855 (TEF1). MycoBank accession number for _Rhodotorula frigidialcoholis _is MB 835866. Diagnosis: After a 14-day
incubation at 15 °C on 5% MEA agar, the streaked cultures are slimy, liquid and shinning with entire margin, greyish red (7B6) (Fig. 1). The cells are subglobose, 3.5 × 4.5 µm, and
encapsulated, budding is unipolar (Fig. 1). Fermentation was not observed. Assimilated compounds are D-glucose, sucrose, raffinose, galactose, trehalose, maltose, melezitose, L-sorbose,
L-rhamnose, D-xylose, Dl-arabinose, D-ribose, glycerol, galactitol, D-mannitol, and xylitol. Not observed assimilated compounds are inulin, melibiose, lactose, methyl A-D-glucoside,
cellobiose, erythritol, myo-inositol, citrate, D-glucosamine, N-acetyl-D-glucosamine, nitrate, and nitrite. Hydrolysis of urea is positive. A complete list of assimilated and not assimilated
compounds is listed in the Supplementary Table S1. Growth from 0 to 37°C (Supplementary Fig. S4). Strain JG1b was previously described as able to grow as low as −10 °C [1, 24], but appears
to have lost subzero growth capabilities through sub-culturing and long-term storage at −80 °C, as in this study _R. frigidialcoholis_ was only successfully grown at 0 °C while incubated in
a PDB 50 ml liquid culture, without shaking. An extended description of _Rhodotorula frigidialcoholis _ is detailed in the Supplementary material (Supplementary file 1) a complete list of
assimilated and not assimilated compounds in the Table S1. PHENOTYPIC MICROARRAY RESPONSE AT COLD TEMPERATURES To characterize _R. frigidialcoholis_ metabolic capabilities at low
temperature, its substrate utilization was assessed through the Biolog Phenotypic MicroArray that determines the ability of a microorganism to utilize a variety of sole carbon or nitrogen
sources [41]. Positive detection of metabolic activity relies on the transfer of electrons from the growth substrate, through the electron transport chain (ETC), to the tetrazolium redox
dye, transforming the dye into a violet colored reduced form [41, 42]. After 91 days of incubation, _R. frigidialcoholis_ utilized 35 on 190 substrates as a sole carbon source at 0 °C for
growth (Supplementary Table S2). Only two (L-Proline, L-Pyroglutamate) of the 95 nitrogen sources were utilized as sole nitrogen sources at 0 °C after 91 days of incubation (Supplementary
Table S2). Surprisingly, of the 35 positive carbon substrates, only six (d-Trehalose, d,l-α-Glycerol Phosphate, d-Mannose, Tween 20, Tween 40, and Tween 80) showed reduction of the dye and
parallel evidence of microbial growth (Supplementary Table S2). This was puzzling because in the remaining 29 carbon sources, we observed active growth in the wells (based on OD600 and
visual inspection) but without reduction of the dye; this growth was confirmed by streak plates. As the tetrazolium dye is reduced via the ETC [43], the observation of growth but no
reduction of the dye suggest that at 0 °C _R. frigidialcoholis_ potentially produces most of the energy for cellular growth through metabolic pathways other than ETC, such as glycolysis or
perhaps fermentation. Damage to the mitochondrial DNA and the ETC in _S. cerevisiae_ was previously shown to decrease tetrazolium dye reduction [44] while yeast mitochondria mutants have
respiratory dysfunction and an inability to reduce tetrazolium salts [45,46,47]. Therefore, growth of _R. frigidialcoholis_ coupled with a lack of tetrazolium dye reduction suggests that
cell respiration through the mitochondria and ETC was significantly reduced while growth was still maintained at 0 °C. MRNA TRANSCRIPTIONAL RESPONSES TO COLD TEMPERATURE To determine _R.
frigidialcoholis_ transcriptional response to cold, we performed comparative mRNA transcriptomic analysis on triplicate exponential phase cultures of _R. frigidialcoholis_ grown at 0 and 23
°C. Overall, 1772 genes were significantly differentially expressed (_p_ < 0.05) between these two conditions, including 994 genes overexpressed, and 778 genes downregulated at 0 °C
compared to 23 °C (Supplementary Tables S3 and S4). Of these significantly differentially expressed genes, 52% were annotated with KEGG. The major global differences in transcript abundance
between 0 and 23 °C were found in genes related to carbohydrate metabolism, energy metabolism, lipid metabolism and signal transduction, glycan biosynthesis/metabolism, transcription
machinery, translation, and amino acid metabolism (Fig. 2). While protein levels are not always correlated with the transcript levels as they are also influenced by post-transcriptional,
translational, and degradation regulation, mRNA levels do partially correlate with protein abundance [48] and are informative to deduce overall metabolic processes [49]. CELLULAR MEMBRANE
AND SIGNAL TRANSDUCTION Maintenance in membrane fluidity through lipid membrane component modification is one of the best-known adaption strategies in microorganisms living in cold
environments [23]. Overall, _R. frigidialcoholis_ overexpressed genes involved in lipid metabolism at 0 °C compared to 23 °C (Figs 2 and 3 and Supplementary Table S5). Similar to previous
studies, genes related to unsaturated fatty-acids (FA) biosynthesis were overall overexpressed at 0 °C indicating increased unsaturated FA concentrations maintain membrane fluidity at cold
temperatures [50,51,52,53]. Relatively high amounts of unsaturated FAs were also reported in other psychrophilic basidiomycetous yeasts isolated from Antarctic and Patagonian ecosystems
[54,55,56]. Sphingolipid metabolism genes were also highly overexpressed at 0 °C compared to 23 °C in _R. frigidialcoholis_ (Supplementary Fig. S4) presumably leading to increased
biosynthesis of ceramide and phytoceramide. Phytoceramide synthesis genes were previously shown to be consistently up-regulated during low temperatures fermentation (12 °C) in _Saccharomyces
cerevisiae_ [57, 58]. Ceramide and other sphingolipid products can induce cell cycle arrest [59] indicating growth suppression in _R. frigidialcoholis_ may be a response to freezing
conditions in Antarctic permafrost habitats. Multiple _Rhodotorula_ species produce lipid antioxidants and photoprotective carotenoids [60,61,62], especially high concentrations of
β-carotene, torulene, and torularhodin. Carotenoids help maintain membrane rigidity in cold-adapted microorganisms as they balance out the higher percentage of unsaturated FAs [50, 63]. At 0
°C, _R. frigidialcoholis_ overexpressed the phytoene desaturase gene (FC = 4.46, _p_ = 0.00) involved in the production of torulene and β-carotene precursors. This is corroborated by our
observation of a darker pink color in _R. frigidialcoholis_ grown at 0 °C compared to 23 °C. Carotenoids play an important role in UV and sunlight protection in yeast [61, 64, 65]. Thus, at
0 °C, _R. frigidialcoholis_ may increase carotenoid biosynthesis both to regulate membrane fluidity and as an adaption to solar irradiation or long-term background γ radiation in the extreme
University Valley permafrost environment. The PI3k-Akt, Hippo, TGFß, VEGF, RAS, MAPK, RAP1, FoxO, and Wnt cell signal transduction pathways genes were overexpressed at 0 °C (Fig. 2 and
Supplementary Table S4); these pathways are related to gene regulation, actin cytoskeleton formation, and cellular adhesion, and are activated by transmembrane receptors. While little is
known about cold response signal transduction modulation, it has been linked to freezing temperature survival in plants [66, 67]. Perception of cold in _Rhodotorula_ by cold-sensitive
membrane receptors may also lead to a cascade response, thus preparing it for cold temperatures. In yeasts, mitogen activated protein kinase cascades play major roles in gene transcription
and actin cytoskeletal organization [68]. Thus, _R. frigidialcoholis_ may overexpress genes coding for the formation of the actin cytoskeleton (cdc42, actin δ/γ subunits) at 0 °C
(Supplementary Table S4) as a way to transport lipids (biosynthesis of which is also overexpressed at 0 °C) to the membrane and maintain membrane fluidity (Figs 2 and 3 and Supplementary
Fig. S4). DIFFERENTIAL EXPRESSION OF HOMOLOGOUS PROTEINS BETWEEN 0 AND 23 °C We observed 19 differentially expressed homologous protein categories between _R. frigidialcoholis_ cultures at 0
and 23 °C (Supplementary Table S6). Eight of these differentially expressed homologous protein categories were related to membrane structure and transport across membranes. For example,
YAT, an amino acid transporter, had three homolog genes overexpressed at 0 °C, two homologs overexpressed at 23 °C, and two homologs that were not differentially expressed between the two
temperatures (Supplementary Table S6). Homologous proteins arise from gene duplication and sometimes gain new functions [69]. Gene duplication, genome redundancy, and paralogous (homologs)
genes increase the organism’s ability to grow under varied conditions (e.g., temperatures, substrates, pH, salinity) [2, 70, 71]. GLYCAN BIOSYNTHESIS AND METABOLISM A significant increase in
glycan biosynthesis genes was observed in _R. frigidialcoholis_ at 0 °C compared to 23 °C (Figs 2 and 3). Glycans are carbohydrate-based polymers associated with cellular protection and
storage [72] and are also important component of glycoproteins, including cell-surface membrane proteins, such as receptors and adhesion proteins [72, 73]. _Rhodotorula_ species produce
mannan, a mannose glycan extracellular polysaccharide (EPS) [74, 75]. Microbial EPS are components of microbial biofilms and constitute a protective matrix against the desiccation and
environmental fluctuations [76, 77], including freeze-thaw damage [78]. At 0 °C, _R. frigidialcoholis_ overexpressed genes involved in GDP-D-mannose synthesis (a glycan biosynthesis
precursor), and overexpressed multiple genes coding for glycosyltransferase and glycosidases (Supplementary Table S4), which regulate glycans [79]. These results indicate that _R.
frigidialcoholis_ increases EPS synthesis at 0 °C through overexpression of mannan and other glycoproteins as adaptation to desiccation and freeze-thaw cycles of the Antarctic University
Valley permafrost environment. TRANSCRIPTION, TRANSLATION, AND AMINO ACID METABOLISM _R. frigidialcoholis_ overexpressed multiple genes involved in the transcriptional machinery at 0 °C
compared to 23 °C (Figs 2 and 3), including the overexpression of genes coding for the three RNA polymerase complexes (RNA Polymerase I, II, and III and the RNA polymerase II transcription
factor) (Supplementary Table S4). A similar increase in the transcriptional machinery at 10 and 4 °C was reported in other yeasts [80,81,82,83]. Contrariwise, _R. frigidialcoholis_ decreased
expression of genes related to translation at 0 °C compared to 23 °C, such as the downregulation of genes involved in ribosomal protein synthesis and aminoacyl-tRNAs. In addition, _R.
frigidialcoholis_ overexpressed proteasome genes involved in protein degradation at 0 °C. Cold temperatures can reduce translation efficiency and protein synthesis by inducing formation of
secondary structures in DNA/RNA molecules and by inactivating ribosomes [4, 83, 84]. While amino acid biosynthesis genes were overall downregulated at 0 °C compared to 23 °C (Fig. 2), the
bacterium _Polaromonas_ sp. also downregulated genes involved in amino acid biosynthesis and transport [8]. Thus, at low temperatures, _R. frigidialcoholis_ may decrease amino acid synthesis
and transport to slow down translation and focus energy on cold acclimation, in parallel with increased protein degradation to possibly recycle amino acids. A notable exception was of
upregulation of the histidine pathway; this could be due to its role in membrane cold sensor histidine kinases [85] as was reported in the basidiomycetous yeast _Mrakia blollopis_, which
accumulated aromatic amino acids, such as histidine, at −3 °C compared to 10 °C [86]. CARBOHYDRATE METABOLISM AND ENERGY METABOLISM The majority of carbohydrate metabolism expression
differences between 0 and 23 °C were in genes of the citrate cycle, pentose phosphate pathway (PPP), and alcohol fermentation. At 23 °C, _R. frigidialcoholis_ significantly overexpressed
citrate cycle genes as well as glycolysis genes linked to oxaloacetate and acetyl-CoA syntheses which feed into the citrate cycle (Fig. 4 and Supplementary Table S5). In contrast, at 0 °C,
there was a significant increase in the expression of glycolysis genes feeding into ethanol production via fermentation and a significant increase in expression of the PPP genes (Fig. 4). At
0 °C, _R. frigidialcoholis_ overexpressed genes coding for xylulose reductase (FC = 2.40, _p_ = 0.00), non-oxidative PPP enzymes, and alcohol dehydrogenase (FC = 8.05, _p_ = 0.00), while it
downregulated gene coding for 6-phosphogluconate dehydrogenase (FC = 2.21, _p_ = 0.00) (Fig. 4). The increase in PPP promotes the production of erythrose-4-phospate, allowing energy (NADH)
conservation due to a reduction of the citrate cycle activity [87]. In yeast, including _Rhodotorula_, the PPP is involved in xylulose fermentation [88,89,90], while downregulation of
6-phosphogluconate dehydrogenase activity is correlated with increased production of ethanol via xylulose fermentation [91]. Xylulose is fermented by xylulose reductase [88] to xylitol, a
cryoprotectant and freezing point depressant in yeast [92]. The non-oxidative PPP enzymes can also increase the yield of ethanol fermentation through the conversion of pentose phosphate into
intermediates of the glycolysis pathway [90]. A similar upregulation of alcohol dehydrogenase was also observed in the Arctic permafrost bacterium, _Planococcus halocryophilus_, grown at
−15 °C [2]. These transcriptional changes in _R. frigidialcoholis_ suggest that it adapts to cold temperature by increasing xylitol production and redirecting PPP molecules to ethanol
fermentation, which would be beneficial for its survival in extreme cryoenvironments such as Antarctic University Valley. Antioxidant production through proline-linked PPP was proposed in
yeast [93] as an oxidative stress response to cold [87]. _R. frigoalchoholis_ can use L-Proline as a sole carbon and nitrogen source (Supplementary Table S2), without reducing the
tetrazolium redox dye, suggesting that L-Proline is used by _Rhodotorula_ in the proline-linked PPP, leading to decreased citrate cycle activity. This may result in a decrease of the ETC
activity and, consequently, the observed lack of tetrazolium dye reduction and a possible redirection of erythrose-4-phosphate to the fermentation. Overall, increased transcription of the
PPP genes at cold temperatures could help _R. frigidialcoholis_ to conserve NADH conservation, limit carbon loss through CO2, and enhance ethanol production to lower the freezing point.
Overall, a higher number of genes related to energy metabolism were downregulated at 0 °C compared to 23 °C (Fig. 2). In accordance with our carbohydrate metabolism results, this significant
change was related to an overall downregulation of the genes encoding for the ETC subunits at 0 °C (Fig. 5). The temperature effect on the ETC activity remains unclear, but some studies
confirm a general trend in decrease of ETC expression at lower temperatures in microorganisms [8, 94]. The University Valley permafrost bacterium, _Rhodococcus_ JG3, also downregulated
multiple ETC cytochromes when grown at −5 °C compared to 23 °C [8], and _Pseudomonas putida_ downregulated its NADH and ETC succinate dehydrogenase at 10 °C compared to 30 °C [94]. The ETC
gene expression patterns in _R. frigidialcoholis_ supports our hypothesis related to the lack of dye reduction in the PM assay at 0 °C as being due to the downregulation of the ETC and a
switch to fermentative metabolisms to produce energy. This is further supported by the observed switch in gene expression from the citrate cycle pathway at 23 °C to the fermentation ethanol
and xylitol pathways at 0 °C for primary energy production (Fig. 4). ETHANOL PRODUCTION IN _RHODOTORULA FRIGIDIALCOHOLIS_ _R. frigidialcoholis’_ switch in metabolism from mainly respiratory
at 23 °C to fermentative at 0 °C was experimentally confirmed as ethanol production was observed at 0 °C, at an average rate of 1.51 × 10−5 moles of ethanol, per liter, per day (Fig. 6A),
while ethanol production was not detected at 23 °C (Fig. 6B). Other _Rhodotorula_ species, _R. minuta, R. mucilaginosa_, and _R. pallida_, are capable of ethanol fermentation at 28 °C [95];
however, ethanol fermentation at low temperatures in _Rhodotorula_ has not been reported. Yeast ethanol production at cold temperatures was reported with _Saccharomyces cerevisiae_ in wine
(0 and 2 °C) and beer (6 °C) production, although in these studies, fermentation was facilitated by an addition of biocatalysts [96,97,98]. To our knowledge, _R. frigidialcoholis_ is able to
naturally produce ethanol at the lowest recorded temperature for microbial fermentation. _R. frigidialcoholis_ could then be studied on its potential to isolate cold-temperature active
enzymes that promote cellulosic biomass decomposition through fermentation [99], favorizing biofuel production with less energy. Ethanol fermentation has also previously been linked with an
increased production of carotenoids in yeast [100], which is consistent with _R. frigidialcoholis_ transcriptomic results of increased carotenoid gene expression. Combined together,
downregulation of citrate cycle and ETC genes (Figs 4 and 5), overexpression of fermentation genes (Fig. 4), growth without reduction of the tetrazolium redox dye (Supplementary Table S2),
and ethanol production at 0 °C but not at 23 °C suggest that _R. frigidialcoholis_ switches from a mainly respiratory metabolism when incubated at 23 °C to a mainly fermentative metabolism
when cultured in conditions similar to its natural environment at 0 °C. Ethanol production in _R. frigidialcoholis_ may be an ecologically significant adaptive response to subzero
temperatures by both acting as a freezing point depressant reducing intracellular freezing and/or to decrease its environment freezing point enhancing the formation of subzero liquid brine
vein microhabitats thought to exist within permafrost [101]. Ethanol production could also promote NADH conservation and limiting carbon loss by switching from the citrate cycle to ethanol
fermentation. This consequently may increase its survivability in the extremely cold Antarctic permafrost habitat from which it was isolated (Fig. 3). SMALL NON-CODING RNA EXPRESSION CHANGES
UNDER COLD TEMPERATURE Small non-coding RNA (<200 nucleotides; sRNA) have numerous functions, including protein synthesis (small rRNAs and tRNAs) and mRNA regulation through RNA
interference (RNAi) [102]. The roles of sRNA and RNAi are not well understood, but they have been linked to stress response [103, 104]. Based on quantification of the total RNA extracted
from _R. frigidialcoholis_ cultures, we initially observed a significantly higher (_p_ = 0.0003) proportion of sRNAs (<200 nt) in the 0 °C compared to 23 °C cultures (Supplementary Fig.
S3). To determine how the sRNA were affected by low temperature in _R_. _frigoalcoholis_, we further analyzed the composition of sRNAs at 0 and 23 °C. On average, 53.40 ± 0.99% of the total
RNA was characterized as sRNAs in 0 °C stressed cultures compared to 27.70 ± 1.84% in 23 °C cultures. There are multiple types of sRNA molecules including miRNAs that are non-coding ~22 nt
sequences that play a role in gene regulation of eukaryote cells, including fungi [105, 106], and rRFs that are derived from rRNA [107]. We subsequently observed a higher proportion (_p_ =
0.029) of short rRFs and miRNA (15–30 nt) in _R. frigoalcoholis_ at 0 °C compared to 23 °C (Supplementary Fig. S3). Specifically, 0.69 ± 0.34% of the total RNA was characterized as short
rRF/miRNAs in 0 °C stressed cultures compared to 0.02 ± 0.01% at 23 °C, resulting in a ~34-fold increase at 0 °C. In addition, we observed a higher diversity in short rRF/miRNAs at 0 °C
compared to 23 °C, based on PCoA analysis (Fig. 7 and Supplementary Fig. S3). Stability and non-random sequence distribution of rRFs in previous studies implies that they are not merely
products of rRNA turnover, but a functional part of cellular homeostasis, although they have historically been eliminated from sequencing datasets as contaminants [107]. rRFs can be produced
from stressed induced cleavage of tRNAs and rRNAs [107, 108] and globally downregulate translation by binding to ribosomes [109, 110] or by binding to mRNA, both triggering RNAi [107, 111,
112]. Thus, rRF/miRNAs production may be a stress response resulting in overall slower growth rate and metabolism. Strong links between cold adaptation in prokaryotes and fungi through miRNA
regulation have not yet been reported, although this mechanism was identified as a cold adaptation in plants [103]. rRFs have also been linked to cold and UV stress [107], and inhibition of
protein translation following DNA damage [113]. Another subset of rRFs are antisense ribosomal small interfering RNAs (risiRNAs) that downregulate pre-rRNA levels through the nuclear RNAi
pathway under cold and UV stress to maintain rRNA homeostasis [114, 115]. This phenomenon is triggered by production of erroneous rRNAs transcripts due to environmental stress [116]. Thus,
this RNAi pathway potentially decreases global translation by targeting rRNA thus slowing down the cell cycle and limiting production of erroneous proteins. We could not find any studies
clearly reporting the percent of rRFs/miRNAs of total RNA in microorganisms to compare with our results. However, we postulate that the 34-fold increase of rRF/miRNAs at 0 °C in _R.
frigidialcoholis_ may be a cold temperature response and result from a combination of three possible RNAi mechanisms (Fig. 3): (1) the rRFs were derived from rDNA triggered by DNA damage
[113, 115] due to cold stress, resulting in global suppression of mRNA translation, and consequently allowing _R. frigidialcoholis_ to slow down its cell cycle under cold temperature; (2)
the rRFs observed could act via the nuclear RNAi pathway to downregulate pre-rRNA levels thus suppressing global translation via a negative feedback loop that targets immature rRNA. This
pathway may be triggered by an increase in errors during rRNA transcription due to environmental stresses such as low temperature [114]. At 0 °C, we observed rRFs with sequence variability
suggesting that erroneous rRNA were potentially produced at a higher rate in _R. frigidialcoholis_ at 0 °C than 23 °C. (3) _R. frigidialcoholis_ may contain miRNA derived from mRNA and short
rRFs derived from rRNA. Both of these could target and suppress a variety of targeted mRNAs in response to cold, as previously observed in plants [103]. Using a RNAhybrid computational
method [40], the top putative mRNA targets for the ten most expressed short rRFs/miRNAs (Table 1) were predicted, based on the lowest energy of hybridization required to form a duplex [106].
Of the ten most abundant short rRFs/miRNAs, nine appear to originate from rRNA and one from a hypothetical protein (Table 1). These short rRFs/miRNAs were mainly overexpressed at 0 °C in
_R. frigidialcoholis_ and potentially target a wide variety of mRNAs as summarized in Table 1. However, we could not directly correlate the increase in expression of the short rRFs/miRNAs
with a decrease in expression of their putative mRNA targets, nor do the targets appear to be necessarily linked to cold adaptation. Absence of correlation between specific mRNA target
expression levels and short rRFs/miRNAs levels could potentially be due to (a) multiple targets per short rRFs/miRNAs, (b) short rRFs/miRNAs targeting rRNAs (instead of mRNAs) that results
in an overall global reduction of translation, or (c) RNAi only partially contributing to gene expression regulation. Through the action of two RNAse III-type proteins, Drosha and Dicer,
miRNAs are produced and recognize specific “target” mRNA that are then silenced via RNAi. Drosha is responsible for the primary transcript cleavage to create precursor miRNA, while Dicer,
along with Argonaute (Ago), cleaves the precursor miRNA to form a double strand miRNA. One of these miRNA strands is incorporated into the RNA-induced silencing complex that suppresses
translation [106]. Short rRFs have been associated with Ago proteins suggesting that rRFs may act like ribosomal derived miRNA as well [112]. Based on the transcriptomic results, the gene
coding for Drosha was not differentially expressed; however, Dicer (FC = 3.83, _p_ = 0.01) and RNA polymerase III subunits were significantly overexpressed at 0 °C (Supplementary Table S4).
This was consistent with a significantly higher proportion of short rRFs/miRNAs at 0 °C compared to 23 °C and a decrease in the transcript levels of the translational machinery (ribosome
proteins and tRNAs) at 0 °C (Fig. 2). Taken together, these results suggest that _R. frigidialcoholis_ may induce a short rRFs/miRNAs gene regulatory mechanism in response to cold that
triggers translational repression; however, molecular characterizations of the short rRFs/miRNAs, such as stem-loop RT-qPCR and northern blot analysis, are necessary to confirm the
involvement of RNAi in cold adaptation. CONCLUSIONS Transcriptomic analyses results suggest that _Rhodotorula frigidialcoholis_ adapts to cold temperatures in the Antarctic dry valley
permafrost through a variety of mechanisms including increasing expression of the PPP genes, increasing the production of carotenoids, sphingolipids, unsaturated fatty acid, and
exopolysaccharides while coupled with a reduction in expression of growth, transcriptional and translational machinery genes. We also identified novel cold adaptation features including a
switch from respiratory metabolism at 23 °C to fermentative metabolism when grown in a cold condition (0 °C), via downregulation of citrate cycle genes and the ETC genes. In parallel, _R.
frigidialcoholis_ overexpressed genes involved in ethanol and xylitol fermentation, resulting in ethanol production at the lowest known temperature described so far in any microorganism.
This switch may thus be an adaptation to save energy and delay intracellular freezing in cryoenvironments, such as the Antarctic permafrost. At low temperature, _R. frigidialcoholis_ also
produced a significantly higher proportion of sRNAs, specifically short rRFs/miRNAs, and overexpressed the Dicer gene, suggesting RNAi as a novel mechanism of cold adaptation in polar fungi.
Further characterization of the role of ethanol production and identification of mRNA targets of RNAi are needed to determine the roles they play in cold adaptation of yeasts inhabiting the
cryosphere. Taken together, our results indicate that _R. frigidialcoholis_ has evolved multiple mechanisms to survive in one of the coldest and driest cold environments on Earth.
REFERENCES * Goordial J, Davila A, Lacelle D, Pollard W, Marinova MM, Greer CW, et al. Nearing the cold-arid limits of microbial life in permafrost of an upper dry valley, Antarctica. ISME
J. 2016;10:1613. Article PubMed PubMed Central Google Scholar * Mykytczuk NC, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. Bacterial growth at −15 C; molecular insights from the
permafrost bacterium _Planococcus halocryophilus_ Or1. ISME J. 2013;7:1211. Article CAS PubMed PubMed Central Google Scholar * Margesin R, Miteva V. Diversity and ecology of
psychrophilic microorganisms. Res Microbiol. 2011;162:346–61. Article PubMed Google Scholar * De Maayer P, Anderson D, Cary C, Cowan DA. Some like it cold: understanding the survival
strategies of psychrophiles. EMBO Rep. 2014;15:508–17. Article PubMed PubMed Central Google Scholar * Hassan N, Rafiq M, Hayat M, Shah AA, Hasan F. Psychrophilic and psychrotrophic
fungi: a comprehensive review. Rev Environ Sci Bio. 2016;15:147–72. Article Google Scholar * Christner BC, Mosley‐Thompson E, Thompson LG, Reeve JN. Bacterial recovery from ancient glacial
ice. Environ Microbiol. 2003;5:433–6. Article CAS PubMed Google Scholar * Raymond-Bouchard I, Goordial J, Zolotarov Y, Ronholm J, Stromvik M, Bakermans C, et al. Conserved genomic and
amino acid traits of cold adaptation in subzero-growing Arctic permafrost bacteria. FEMS Microbiol Ecol. 2018;94:fiy023. Article Google Scholar * Raymond-Bouchard I, Tremblay J, Altshuler
I, Greer CW, Whyte LG. Comparative transcriptomics of cold growth and adaptive features of a eury-and steno-psychrophile. Front Microbiol. 2018;9:1565. Article PubMed PubMed Central
Google Scholar * Buzzini P, Margesin R. Cold-adapted yeasts: a lesson from the cold and a challenge for the XXI century. In: Buzzini P, Margesin R, editors. Cold-adapted yeasts. Heidelberg:
Springer; 2014. p. 3–22. Chapter Google Scholar * Altshuler I, Goordial J, Whyte LG. Microbial life in permafrost. In: Margesin R, editor. Psychrophiles: from biodiversity to
biotechnology. 2nd edn. Cham: Springer; 2017. p. 153–79. Chapter Google Scholar * Gilichinsky D, Wilson G, Friedmann E, McKay C, Sletten R, Rivkina E, et al. Microbial populations in
Antarctic permafrost: biodiversity, state, age, and implication for astrobiology. Astrobiology. 2007;7:275–311. Article CAS PubMed Google Scholar * de Menezes GCA, Porto BA, Amorim SS,
Zani CL, de Almeida Alves TM, Junior PAS, et al. Fungi in glacial ice of Antarctica: diversity, distribution and bioprospecting of bioactive compounds. Extremophiles. 2020;24:367–76. Article
PubMed Google Scholar * Zhang T, Wang N, Yu L. Soil fungal community composition differs significantly among the Antarctic, Arctic, and Tibetan Plateau. Extremophiles. 2020;24:821–9.
Article CAS PubMed Google Scholar * Coleine C, Zucconi L, Onofri S, Pombubpa N, Stajich JE, Selbmann L. Sun exposure shapes functional grouping of fungi in cryptoendolithic Antarctic
communities. Life. 2018;8:19. Article PubMed Central Google Scholar * Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitaš A. Hypersaline waters in salterns–natural ecological niches for
halophilic black yeasts. FEMS Microbiol Ecol. 2000;32:235–40. CAS Google Scholar * Perini L, Gostinčar C, Anesio AM, Williamson C, Tranter M, Gunde-Cimerman N. Darkening of the Greenland
Ice Sheet: fungal abundance and diversity are associated with algal bloom. Front Microbiol. 2019;10:557. Article PubMed PubMed Central Google Scholar * Tojo M, Newsham KK. Snow moulds in
polar environments. Fungal Ecol. 2012;5:395–402. Article Google Scholar * Rosa LH, Vaz AB, Caligiorne RB, Campolina S, Rosa CA. Endophytic fungi associated with the Antarctic grass
_Deschampsia antarctica_ Desv.(Poaceae). Polar Biol. 2009;32:161–7. Article Google Scholar * Gianoli E, Inostroza P, Zúñiga-Feest A, Reyes-Díaz M, Cavieres LA, Bravo LA, et al. Ecotypic
differentiation in morphology and cold resistance in populations of Colobanthus quitensis (Caryophyllaceae) from the Andes of central Chile and the maritime Antarctic. Arct Antarct Alp Res.
2004;36:484–9. Article Google Scholar * Duncan SM, Farrell RL, Thwaites JM, Held BW, Arenz BE, Jurgens JA, et al. Endoglucanase‐producing fungi isolated from Cape Evans historic expedition
hut on Ross Island, Antarctica. Environ Microbiol. 2006;8:1212–9. Article CAS PubMed Google Scholar * Starmer WT, Lachance M-A. Yeast ecology. In: Kurtzman CP, Fell JW, Boekhout T, eds.
The yeasts. 5ft ed. London: Elsevier; 2011. p. 65–83. Chapter Google Scholar * Shivaji S, Prasad G. Antarctic yeasts: biodiversity and potential applications. In: Satyanarayana T, Kunze
G, editors. Yeast biotechnology: diversity and applications. New Delhi: Springer; 2009. p. 3–18. Chapter Google Scholar * Gunde-Cimerman N, Plemenitaš A, Buzzini P. Changes in lipids
composition and fluidity of yeast plasma membrane as response to cold. In: Buzzini P, Margesin R, editors. Cold-adapted yeasts. Heidelberg: Springer; 2014. p. 225–42. Chapter Google Scholar
* Goordial J, Raymond-Bouchard I, Riley R, Ronholm J, Shapiro N, Woyke T, et al. Improved high-quality draft genome sequence of the eurypsychrophile Rhodotorula sp. JG1b, isolated from
permafrost in the hyperarid upper-elevation mcmurdo dry valleys, Antarctica. Genome Announc. 2016;4:e00069–16. Article PubMed PubMed Central Google Scholar * Yen H-W, Liao Y-T, Liu YX.
Cultivation of oleaginous _Rhodotorula mucilaginosa_ in airlift bioreactor by using seawater. J Biosci Bioeng. 2016;121:209–12. Article CAS PubMed Google Scholar * Buzzini P, Turk M,
Perini L, Turchetti B, Gunde-Cimerman N. Yeasts in polar and subpolar habitats. In: Buzzini P, Lachance M-A, Yurkov A, editors. Yeasts in natural ecosystems: diversity. Cham: Springer; 2017.
p. 331–65. Chapter Google Scholar * Margesin R, Fonteyne P-A, Schinner F, Sampaio JP. _Rhodotorula psychrophila_ sp. nov., _Rhodotorula psychrophenolica_ sp. nov. and _Rhodotorula
glacialis_ sp. nov., novel psychrophilic basidiomycetous yeast species isolated from alpine environments. Int J Syst Evol Micr. 2007;57:2179–84. Article CAS Google Scholar * Sabri A,
Jacques P, Weekers F, Bare G, Hiligsmann S, Moussaif M, et al. Effect of temperature on growth of psychrophilic and psychrotrophic members of _Rhodotorula aurantiaca_. In: Walt DR, editor.
Applied biochemistry and biotechnology. New York: Springer Science+Business Media; 2000. p. 391–9. Google Scholar * Marchant DR, Head JW III. Antarctic dry valleys: microclimate zonation,
variable geomorphic processes, and implications for assessing climate change on Mars. Icarus 2007;192:187–222. Article Google Scholar * Kurtzman C, Fell JW, Boekhout T, editors. The
yeasts: a taxonomic study. 5ft ed. London: Elsevier; 2011. Google Scholar * Kornerup A, Wanscher JH, editors. Methuen handbook of colour. 2nd ed. London: Methuen and Co.; 1967. Google
Scholar * Xing W, Yin M, Lv Q, Hu Y, Liu C, Zhang J. Oxygen solubility, diffusion coefficient, and solution viscosity. In: Xing W, Yin G, Zhang J, editors. Rotating electrode methods and
oxygen reduction electrocatalysts. London: Elsevier; 2014. p. 1–31. Google Scholar * Viti C, Decorosi F, Marchi E, Galardini M, Giovannetti L. High-throughput phenomics. In: Mengoni A,
Galardini M, Fondi M, editors. Bacterial pangenomics. Methods and protocols. New York: Springer; 2015. p. 99–123. Chapter Google Scholar * Rico A, Preston GM. _Pseudomonas syringae_ pv.
tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol Plant Microbe. 2008;21:269–82.
Article CAS Google Scholar * Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417.
Article CAS PubMed PubMed Central Google Scholar * Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Article PubMed PubMed Central Google Scholar * Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences.
J Mol Biol. 2016;428:726–31. Article CAS PubMed Google Scholar * Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Article CAS PubMed Google Scholar * Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–54. Article PubMed PubMed
Central Google Scholar * Rehmsmeier M, Steffen P, Höchsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–17. Article CAS PubMed PubMed
Central Google Scholar * Greetham D. Phenotype microarray technology and its application in industrial biotechnology. Biotechnol Lett. 2014;36:1153–60. Article CAS PubMed Google Scholar
* Bochner BR. Global phenotypic characterization of bacteria. FEMS Microbiol Rev. 2008;33:191–205. Article PubMed Google Scholar * Maldonado F, Packard T, Gómez M. Understanding
tetrazolium reduction and the importance of substrates in measuring respiratory electron transport activity. J Exp Mar Biol Ecol. 2012;434:110–8. Article Google Scholar * Barclay BJ,
DeHaan CL, Hennig UG, Iavorovska O, von Borstel RW, Von, et al. A rapid assay for mitochondrial DNA damage and respiratory chain inhibition in the yeast _Saccharomyces cerevisiae_. Environ
Mol Mutagen. 2001;38:153–8. Article CAS PubMed Google Scholar * Jenkins CL, Lawrence SJ, Kennedy AI, Thurston P, Hodgson JA, Smart KA. Incidence and formation of petite mutants in lager
brewing yeast _Saccharomyces cerevisiae_ (syn. S. pastorianus) populations. J Am Soc Brew Chem. 2009;67:72–80. CAS Google Scholar * Glab N, Wise R, Pring D, Jacq C, Slonimski P. Expression
in _Saccharomyces cerevisiae_ of a gene associated with cytoplasmic male sterility from maize: respiratory dysfunction and uncoupling of yeast mitochondria. Mol Gen Genet. 1990;223:24–32.
Article CAS PubMed Google Scholar * Goldring ES, Grossman LI, Krupnick D, Cryer DR, Marmur J. The petite mutation in yeast: loss of mitochondrial deoxyribonucleic acid during induction
of petites with ethidium bromide. J Mol Biol. 1970;52:323–35. Article CAS PubMed Google Scholar * Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic
and transcriptomic analyses. Nat Rev Genet. 2012;13:227–32. Article CAS PubMed PubMed Central Google Scholar * Pinatel E, Peano C. RNA sequencing and analysis in microorganisms for
metabolic network reconstruction. In: Fondi M, editor. Metabolic network reconstruction and modeling. Methods and protocols. New York: Springer; 2018. p. 239–65. Chapter Google Scholar *
Raymond‐Bouchard I, Chourey K, Altshuler I, Iyer R, Hettich RL, Whyte LG. Mechanisms of subzero growth in the cryophile _Planococcus halocryophilus_ determined through proteomic analysis.
Environ Microbiol. 2017;19:4460–79. Article PubMed Google Scholar * Bhuiyan M, Tucker D, Watson K. Gas chromatography–mass spectrometry analysis of fatty acid profiles of Antarctic and
non-Antarctic yeasts. Anton Leeuw. 2014;106:381–9. Article CAS Google Scholar * López-Malo M, Chiva R, Rozes N, Guillamon JM. Phenotypic analysis of mutant and overexpressing strains of
lipid metabolism genes in _Saccharomyces cerevisiae_: implication in growth at low temperatures. Int J Food Microbiol. 2013;162:26–36. Article PubMed Google Scholar * Rossi M, Buzzini P,
Cordisco L, Amaretti A, Sala M, Raimondi S, et al. Growth, lipid accumulation, and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS
Microbiol Ecol. 2009;69:363–72. Article CAS PubMed Google Scholar * Contreras G, Barahona S, Sepúlveda D, Baeza M, Cifuentes V, Alcaíno J. Identification and analysis of metabolite
production with biotechnological potential in _Xanthophyllomyces dendrorhous_ isolates. World J Micro Biot. 2015;31:517–26. Article CAS Google Scholar * Libkind D, Arts M, Van Broock M.
Fatty acid composition of cold-adapted carotenogenic basidiomycetous yeasts. Rev Argent Microbiol. 2008;40:193–7. CAS PubMed Google Scholar * Thomas-Hall S, Watson K. _Cryptococcus
nyarrowii_ sp. nov., a basidiomycetous yeast from Antarctica. Int J Syst Evol Micr. 2002;52:1033–8. CAS Google Scholar * López-Malo M, García-Ríos E, Chiva R, Guillamon JM. Functional
analysis of lipid metabolism genes in wine yeasts during alcoholic fermentation at low temperature. Micro Cell. 2014;1:365. Article Google Scholar * Tai SL, Daran-Lapujade P, Walsh MC,
Pronk JT, Daran J-M. Acclimation of _Saccharomyces cerevisiae_ to low temperature: a chemostat-based transcriptome analysis. Mol Biol Cell. 2007;18:5100–12. Article CAS PubMed PubMed
Central Google Scholar * Mao C, Wadleigh M, Jenkins GM, Hannun YA, Obeid LM. Identification and characterization of _Saccharomyces cerevisiae_ dihydrosphingosine-1-phosphate phosphatase. J
Biol Chem. 1997;272:28690–4. Article CAS PubMed Google Scholar * Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN. Biotechnological production of carotenoids by yeasts: an
overview. Micro Cell Fact. 2014;13:12. Article Google Scholar * Moliné M, Flores MR, Libkind D. del Carmen Diéguez M, Farías ME, van Broock M. Photoprotection by carotenoid pigments in the
yeast Rhodotorula mucilaginosa: the role of torularhodin. Photoch Photobio Sci. 2010;9:1145–51. Article Google Scholar * Liu GY, Nizet V. Color me bad: microbial pigments as virulence
factors. Trends Microbiol. 2009;17:406–13. Article CAS PubMed PubMed Central Google Scholar * Rodrigues DF, Tiedje JM. Coping with our cold planet. Appl Environ Micro. 2008;74:1677–86.
Article CAS Google Scholar * Villarreal P, Carrasco M, Barahona S, Alcaíno J, Cifuentes V, Baeza M. Tolerance to ultraviolet radiation of psychrotolerant yeasts and analysis of their
carotenoid, mycosporine, and ergosterol content. Curr Microbiol. 2016;72:94–101. Article CAS PubMed Google Scholar * Moliné M, Libkind D, del Carmen DiéguezM, van Broock M.
Photoprotective role of carotenoids in yeasts: response to UV-B of pigmented and naturally-occurring albino strains. J Photoch Photobio B 2009;95:156–61. Article Google Scholar * Huang
G-T, Ma S-L, Bai L-P, Zhang L, Ma H, Jia P, et al. Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep. 2012;39:969–87. Article PubMed Google Scholar *
Heino P, Palva ET. Signal transduction in plant cold acclimation. In: Hirt H, Shinozaki K, editors. Plant responses to abiotic stress. Berlin: Springer; 2003. p. 151–86. Chapter Google
Scholar * Storey KB, Storey JM. Signal transduction and gene expression in the regulation of natural freezing survival. In: Storey KB, Storey JM, editors. Protein adaptations and signal
transduction. London: Elsevier; 2001. p. 1–19. Google Scholar * Li W-H, Yang J, Gu X. Expression divergence between duplicate genes. Trends Genet. 2005;21:602–7. Article PubMed Google
Scholar * Vollmers J, Voget S, Dietrich S, Gollnow K, Smits M, Meyer K, et al. Poles apart: arctic and Antarctic Octadecabacter strains share high genome plasticity and a new type of
xanthorhodopsin. Plos One. 2013;8:e63422. Article CAS PubMed PubMed Central Google Scholar * Wagner A. Asymmetric functional divergence of duplicate genes in yeast. Mol Biol Evol.
2002;19:1760–8. Article CAS PubMed Google Scholar * Varki A, Gagneux P. Biological functions of glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. editors.
Essentials of glycobiology. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2017. Google Scholar * Colley K, Varki A, Kinoshita T. Cellular organization of glycosylation.
In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. editors. Essentials of glycobiology. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2017. Google
Scholar * Pavlova K, Panchev I, Hristozova T. Physico-chemical characterization of exomannan from _Rhodotorula acheniorum_ MC. World J Micro Biot. 2005;21:279–83. Article CAS Google
Scholar * Cho DH, Chae HJ, Kim EY. Synthesis and characterization of a novel extracellular polysaccharide by _Rhodotorula glutinis_. Appl Biochem Biotech. 2001;95:183–93. Article CAS
Google Scholar * Flemming HC, Neu TR, Wingender J. The perfect slime. Microbial extracellular polymeric substances (EPS). London: IWA Publishing; 2016. Book Google Scholar * Nichols WW,
Evans MJ, Slack MP, Walmsley HL. The penetration of antibiotics into aggregates of mucoid and non-mucoid _Pseudomonas aeruginosa_. Microbiology. 1989;135:1291–303. Article CAS Google
Scholar * Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M. Production and structural characterization of the exopolysaccharide of the Antarctic fungus _Phoma herbarum_ CCFEE 5080.
Res Microbiol. 2002;153:585–92. Article CAS PubMed Google Scholar * Rini JM, Esko JD. Glycosyltransferases and glycan-processing enzymes. In: Varki A, Cummings RD, Esko JD, Stanley P,
Hart GW, Aebi M, et al. editors. Essentials of glycobiology. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2017. Google Scholar * Strassburg K, Walther D, Takahashi H,
Kanaya S, Kopka J. Dynamic transcriptional and metabolic responses in yeast adapting to temperature stress. Omics. 2010;14:249–59. Article CAS PubMed PubMed Central Google Scholar *
Becerra M, Lombardia L, Gonzalez-Siso M, Rodriguez-Belmonte E, Hauser N, Cerdán M. Genome-wide analysis of the yeast transcriptome upon heat and cold shock. Int J Genomics. 2003;4:366–75.
CAS Google Scholar * Homma T, Iwahashi H, Komatsu Y. Yeast gene expression during growth at low temperature. Cryobiology. 2003;46:230–7. Article CAS PubMed Google Scholar * Sahara T,
Goda T, Ohgiya S. Comprehensive expression analysis of time-dependent genetic responses in yeast cells to low temperature. J Biol Chem. 2002;277:50015–21. Article CAS PubMed Google
Scholar * Schade B, Jansen G, Whiteway M, Entian KD, Thomas DY. Cold adaptation in budding yeast. Mol Biol Cell. 2004;15:5492–502. Article CAS PubMed PubMed Central Google Scholar *
Mikami K, Kanesaki Y, Suzuki I, Murata N. The histidine kinase Hik33 perceives osmotic stress and cold stress in _Synechocystis_ sp. PCC 6803. Mol Microbiol. 2002;46:905–15. Article CAS
PubMed Google Scholar * Tsuji M. Cold-stress responses in the Antarctic basidiomycetous yeast _Mrakia blollopis_. R Soc Open Sci. 2016;3:160106. Article PubMed PubMed Central Google
Scholar * Sarkar D, Bhowmik PC, Kwon Y-I, Shetty K. Clonal response to cold tolerance in creeping bentgrass and role of proline-associated pentose phosphate pathway. Bioresour Technol.
2009;100:5332–9. Article CAS PubMed Google Scholar * Bura R, Vajzovic A, Doty SL. Novel endophytic yeast _Rhodotorula mucilaginosa_ strain PTD3 I: production of xylitol and ethanol. J
Ind Microbiol Biot. 2012;39:1003–11. Article CAS Google Scholar * da Silva TL, Feijão D, Roseiro JC, Reis A. Monitoring _Rhodotorula glutinis_ CCMI 145 physiological response and oil
production growing on xylose and glucose using multi-parameter flow cytometry. Bioresour Technol. 2011;102:2998–3006. Article PubMed Google Scholar * Johansson B, Hahn-Hägerdal B. The
non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in _Saccharomyces cerevisiae_ TMB3001. FEMS Yeast Res. 2002;2:277–82. CAS PubMed Google
Scholar * Eliasson A, Boles E, Johansson B, Österberg M, Thevelein J, Spencer-Martins I, et al. Xylulose fermentation by mutant and wild-type strains of _Zygosaccharomyces_ and
_Saccharomyces cerevisiae_. Appl Microbiol Biot. 2000;53:376–82. Article CAS Google Scholar * Mohamad N, Mustapa Kamal S, Mokhtar M. Xylitol biological production: a review of recent
studies. Food Rev Int. 2015;31:74–89. Article CAS Google Scholar * Shetty K, Wahlqvist M. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical
bio-synthesis and mechanism of action for human health and environmental applications. Asia Pac J Clin Nutr. 2004;13:1–24. CAS PubMed Google Scholar * Fonseca P, Moreno R, Rojo F. Growth
of _Pseudomonas putida_ at low temperature: global transcriptomic and proteomic analyses. Environ Microbiol Rep. 2011;3:329–39. Article CAS PubMed Google Scholar * Rao R, Bhadra B,
Shivaji S. Isolation and characterization of ethanol‐producing yeasts from fruits and tree barks. Lett Appl Microbiol. 2008;47:19–24. Article CAS PubMed Google Scholar * Kourkoutas Y,
Komaitis M, Koutinas A, Kaliafas A, Kanellaki M, Marchant R, et al. Wine production using yeast immobilized on quince biocatalyst at temperatures between 30 and 0 C. Food Chem.
2003;82:353–60. Article CAS Google Scholar * Kanellaki M, Koutinas AA. Low temperature fermentation of wine and beer by cold-adapted and immobilized yeast cells. In: Margesin R, Schinner
F, editors. Biotechnological applications of cold-adapted organisms. Berlin: Springer; 1999. p. 117–45. Chapter Google Scholar * Bakoyianis V, Kanellaki M, Kaliafas A, Koutinas A.
Low-temperature wine making by immobilized cells on mineral kissiris. J Agr Food Chem. 1992;40:1293–6. Article CAS Google Scholar * Tiwari R, Singh S, Shukla P, Nain L. Novel cold
temperature active β-glucosidase from _Pseudomonas lutea_ BG8 suitable for simultaneous saccharification and fermentation. RSC Adv. 2014;4:58108–15. Article CAS Google Scholar * Tang W,
Wang Y, Zhang J, Cai Y, He Z. Biosynthetic pathway of carotenoids in _Rhodotorula_ and strategies for enhanced their production. J Microbiol Biotechn. 2019;29:507–17. Article CAS Google
Scholar * Steven B, Briggs G, McKay CP, Pollard WH, Greer CW, Whyte LG. Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using
culture-dependent and culture-independent methods. FEMS Microbiol Ecol. 2007;59:513–23. Article CAS PubMed Google Scholar * Dozmorov MG, Giles CB, Koelsch KA, Wren JD. Systematic
classification of non-coding RNAs by epigenomic similarity. BMC Bioinforma. 2013;14:S2. Article Google Scholar * Sunkar R, Li Y-F, Jagadeeswaran G. Functions of microRNAs in plant stress
responses. Trends Plant Sci. 2012;17:196–203. Article CAS PubMed Google Scholar * Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell.
2003;113:673–6. Article CAS PubMed Google Scholar * Lau SK, Chow W-N, Wong AY, Yeung JM, Bao J, Zhang N, et al. Identification of microRNA-like RNAs in mycelial and yeast phases of the
thermal dimorphic fungus _Penicillium marneffei_. Plos Negl Trop D. 2013;7:e2398. Article Google Scholar * Zhou Q, Wang Z, Zhang J, Meng H, Huang B. Genome-wide identification and
profiling of microRNA-like RNAs from Metarhizium anisopliae during development. Fungal Biol UK. 2012;116:1156–62. Article CAS Google Scholar * Lambert M, Benmoussa A, Provost P. Small
non-coding RNAs derived from eukaryotic ribosomal RNA. Noncoding RNA 2019;5:16. Article CAS PubMed Central Google Scholar * Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a
conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–103. Article CAS PubMed PubMed Central Google Scholar * Gebetsberger J, Wyss L, Mleczko AM, Reuther J, Polacek N.
A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017;14:1364–73. Article PubMed Google Scholar * Bąkowska-Żywicka K,
Kasprzyk M, Twardowski T. tRNA-derived short RNAs bind to _Saccharomyces cerevisiae_ ribosomes in a stress-dependent manner and inhibit protein synthesis in vitro. FEMS Yeast Res.
2016;16:fow077. Article PubMed PubMed Central Google Scholar * McCool MA, Bryant CJ, Baserga SJ. MicroRNAs and long non-coding RNAs as novel regulators of ribosome biogenesis. Biochem
Soc T. 2020;48:595–612. Article CAS Google Scholar * Wei H, Zhou B, Zhang F, Tu Y, Hu Y, Zhang B, et al. Profiling and identification of small rDNA-derived RNAs and their potential
biological functions. Plos One. 2013;8:e56842. Article CAS PubMed PubMed Central Google Scholar * Lee H-C, Chang S-S, Choudhary S, Aalto AP, Maiti M, Bamford DH, et al. qiRNA is a new
type of small interfering RNA induced by DNA damage. Nature. 2009;459:274–7. Article CAS PubMed PubMed Central Google Scholar * Zhu C, Yan Q, Weng C, Hou X, Mao H, Liu D, et al.
Erroneous ribosomal RNAs promote the generation of antisense ribosomal siRNA. P Natl Acad Sci USA. 2018;115:10082–7. Article CAS Google Scholar * Zhou X, Chen X, Wang Y, Feng X, Guang S.
A new layer of rRNA regulation by small interference RNAs and the nuclear RNAi pathway. RNA Biol. 2017;14:1492–8. Article PubMed PubMed Central Google Scholar * Zhou X, Feng X, Mao H, Li
M, Xu F, Hu K, et al. RdRP-synthesized antisense ribosomal siRNAs silence pre-rRNA via the nuclear RNAi pathway. Nat Struct Mol Biol. 2017;24:258. Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS The authors acknowledge the valuable input from Dr Jennifer Ronholm and Dr Jacqueline Goordial for their critical feedback and for the setting up of the
project, and Dr Barry R. Bochner, from Biolog Inc. for his valuable advice with the Biolog Phenotypic MicroArray tests analysis. The authors acknowledge the support of the McGill Space
Institute. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Natural Resource Sciences, McGill University, Sainte-Anne-de-Bellevue, QC, Canada D. Touchette, I. Altshuler, I.
Raymond-Bouchard & L. G. Whyte * Department of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia C. Gostinčar, P. Zalar & N. Gunde-Cimerman * Lars Bolund
Institute of Regenerative Medicine, BGI-Qingdao, Qingdao, China C. Gostinčar * Agricultural Institute of Slovenia, Ljubljana, Slovenia J. Zajc * NASA Ames Research Center, Moffett Field, CA,
USA C. P. McKay Authors * D. Touchette View author publications You can also search for this author inPubMed Google Scholar * I. Altshuler View author publications You can also search for
this author inPubMed Google Scholar * C. Gostinčar View author publications You can also search for this author inPubMed Google Scholar * P. Zalar View author publications You can also
search for this author inPubMed Google Scholar * I. Raymond-Bouchard View author publications You can also search for this author inPubMed Google Scholar * J. Zajc View author publications
You can also search for this author inPubMed Google Scholar * C. P. McKay View author publications You can also search for this author inPubMed Google Scholar * N. Gunde-Cimerman View author
publications You can also search for this author inPubMed Google Scholar * L. G. Whyte View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS DT
wrote the manuscript, performed all laboratory experiments and data analysis. IA helped with the experimental design, laboratory experiments, data analysis, figure creation, writing and
manuscript editing. CG performed the bioinformatic pipeline analysis of the raw RNASeq data (mRNA). PZ and JZ described the novelty of the species. IR-B helped with experimental design and
manuscript editing. LGW and NG-C provided guidance, advise, and helped with the manuscript editing. CORRESPONDING AUTHOR Correspondence to L. G. Whyte. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FILE 1 SUPPLEMENTARY FILE 2 SUPPLEMENTARY FILE3 TABLES AND FIGURES SUPPLEMENTARY FILE 4 RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Touchette, D., Altshuler, I.,
Gostinčar, C. _et al._ Novel Antarctic yeast adapts to cold by switching energy metabolism and increasing small RNA synthesis. _ISME J_ 16, 221–232 (2022).
https://doi.org/10.1038/s41396-021-01030-9 Download citation * Received: 23 June 2020 * Revised: 17 May 2021 * Accepted: 02 June 2021 * Published: 22 July 2021 * Issue Date: January 2022 *
DOI: https://doi.org/10.1038/s41396-021-01030-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
