Antihypertensive effect of etamicastat in dopamine d2 receptor-deficient mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Abnormalities of the D2R gene (_DRD2_) play a role in the pathogenesis of human essential hypertension; variants of the _DRD2_ have been reported to be associated with hypertension.
Disruption of _Drd2_ (D2−/−) in mice increases blood pressure. The hypertension of D2−/− mice has been related, in part, to increased sympathetic activity, renal oxidative stress, and renal
endothelin B receptor (ETBR) expression. We tested in D2−/− mice the effect of etamicastat, a reversible peripheral inhibitor of dopamine-β-hydroxylase that reduces the biosynthesis of
norepinephrine from dopamine and decreases sympathetic nerve activity. Blood pressure was measured in anesthetized D2−/− mice treated with etamicastat by gavage, (10 mg/kg), conscious D2−/−
mice, and D2+/+ littermates, and mice with the D2R selectively silenced in the kidney, treated with etamicastat in the drinking water (10 mg/kg per day). Tissue and urinary catecholamines
and renal expression of selected G protein-coupled receptors, enzymes related to the production of reactive oxygen species, and sodium transporters were also measured. Etamicastat decreased
blood pressure both in anesthetized and conscious D2−/− mice and mice with renal-selective silencing of D2R to levels similar or close to those measured in D2+/+ littermates. Etamicastat
decreased cardiac and renal norepinephrine and increased cardiac and urinary dopamine levels in D2−/− mice. It also normalized the increased renal protein expressions of ETBR, NADPH oxidase
isoenzymes, and urinary 8-isoprostane, as well as renal NHE3 and NCC, and increased the renal expression of D1R but not D5R in D2−/− mice. In conclusion, etamicastat is effective in
normalizing the increased blood pressure and some of the abnormal renal biochemical alterations of D2−/− mice. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS THE SALT SENSITIVITY OF _DRD4_-NULL MICE IS ASSOCIATED WITH THE UPREGULATIONS OF SODIUM TRANSPORTERS IN KIDNEYS Article 22 May 2024 BLOCKAGE OF UCHL1 ACTIVITY
ATTENUATES CARDIAC REMODELING IN SPONTANEOUSLY HYPERTENSIVE RATS Article 15 June 2020 COMPARISON OF THE EFFECTS OF RENAL DENERVATION AT EARLY OR ADVANCED STAGES OF HYPERTENSION ON CARDIAC,
RENAL, AND ADIPOSE TISSUE PATHOLOGY IN DAHL SALT-SENSITIVE RATS Article Open access 15 February 2024 INTRODUCTION Inhibition of dopamine β-hydroxylase (DBH) may provide significant clinical
improvement in patients suffering from cardiovascular disorders, such as hypertension and chronic heart failure. The rationale for the use of DBH inhibitors is based on their ability to
inhibit the biosynthesis of norepinephrine (NE), via inhibition of the enzymatic hydroxylation of dopamine (DA) [1]. Direct inhibition of sympathetic nerve function by reducing the
biosynthesis of NE, preventing the conversion of DA to NE in sympathetic nerves, and possibly by increasing the release of DA, can induce renal vasodilation, diuresis, and natriuresis.
β-adrenergic blockers are no longer recommended as primary therapy for hypertension except for patients with coexisting conditions, such as coronary heart disease or left ventricular
dysfunction. α-adrenergic blockers are also not recommended as first-line therapy for hypertension because they may be associated with increased incidence of adverse cerebrovascular and
cardiovascular outcomes [2]. Therefore, inhibitors of DBH may provide significant clinical advantages over other drug treatments, especially those that affect the sympathetic nervous system.
Etamicastat [(R)-5-(2-aminoethyl)-1-(6,8-difluorochroman-3-yl)-1,3-dihydroimidazole-2-thione hydrochloride] is a potent, reversible inhibitor of peripheral DBH with limited access to the
brain [1]. In spontaneously hypertensive rats (SHRs) but not in normotensive control rats, oral administration of etamicastat lowered both systolic and diastolic blood pressures in a
dose-dependent manner without affecting the heart rate [3]. Etamicastat, chronically administered in drinking water, also significantly reduced both blood pressure and urinary NE excretion
but increased urinary DA excretion in the SHR [4]. This DBH inhibitor has also been shown to decrease blood pressure in hypertensive patients [5]. The intrarenal dopaminergic system plays an
important role in the normal regulation of renal sodium excretion and blood pressure [6, 7]. Human essential hypertension and some rodent models of genetic hypertension are associated with
decreased renal DA production and receptor function [6, 8]. Both DA D2-like (_Drd2_, _Drd3_, and _Drd4_) and D1-like (_Drd1_ and _Drd5_) receptors have been shown to regulate arterial blood
pressure. Abnormalities of the D2R gene (_DRD2_) play a role in the pathogenesis of human essential hypertension; several variants of the human _DRD2_ have been reported to be associated
with hypertension [9, 10] and disruption (D2−/−) or renal-selective silencing of _Drd2_ in mice increases systolic and diastolic blood pressures [11,12,13] and may cause salt sensitivity
[14]. The hypertension of D2−/− mice has been related to increased sympathetic and vascular smooth muscle endothelin B receptor (ETBR) activities [11], as well as increased reactive oxygen
species (ROS) [12]. In this study, we determined the effects of inhibition of DBH and NE formation with etamicastat on blood pressure in D2−/− mice. We measured blood pressure in conscious
and anesthetized D2−/− mice and D2+/+ littermates after acute- and short-term administration of etamicastat, catecholamine levels in the heart, kidney, and urine, and renal expression of
selected G protein-coupled receptor and enzymes related to and a parameter of ROS production. In addition, we studied the effect of etamicastat on the elevated blood pressure of mice in
which renal cortical _Drd2_ was silenced by the renal subcapsular infusion of _Drd2_-specific small interfering RNA (siRNA), via an osmotic minipump [13]. We also determined the expression
of selected sodium transporters, exchangers, and channels in the kidney of D2−/− mice and D2+/+ littermates before and after treatment with etamicastat. MATERIALS AND METHODS D2
RECEPTOR-DEFICIENT MICE The original F2 hybrid strain (129/SvXC57Bl/6J, Oregon Health Sciences University, Portland) that contained the mutated D2R allele (D2−/−) was backcrossed into
wild-type C57Bl/6J for >20 generations and genotyped [10]. All mice were bred in the Animal Care Facility of the University of Maryland School of Medicine and The George Washington
University School of Medicine & Health Sciences. Male D2−/− mice and D2+/+ littermates fed 0.6% NaCl were studied at 4–6 months of age. All studies were approved by the Animal Care and
Use Committees of the University of Maryland School of Medicine and The George Washington University School of Medicine & Health Sciences. TREATMENT WITH ETAMICASTAT AND BLOOD PRESSURE
MEASUREMENTS ACUTE TREATMENT Etamicastat (10 mg/kg), synthesized in the Department of Chemistry, BIAL - Portela & Cª, S.A. Portugal, with a purity of 99.5%, or vehicle (tap water) was
administered by gastric gavage (200 µL) to D2−/− and D2+/+ mice. The mice were individually housed in metabolic cages for collection of urine samples before measurement of blood pressure.
Blood pressure was measured 9 or 18 h after drug administration. Systolic and diastolic blood pressures were measured (Cardiomax II, Instruments, Columbus, OH) from the aorta, via the
femoral artery, under pentobarbital anesthesia (50 mg/kg, administered intraperitoneally). Blood pressures were recorded 1 h after the induction of anesthesia when blood pressures were
stable. The mice were killed (pentobarbital 100 mg/kg) at the conclusion of the study. The hearts and kidneys were collected, frozen in isopentane at −30 °C on dry ice, and stored at −80 °C
until studied. Tissue and urine catecholamines were quantified, as reported [1, 15, 16]. SHORT-TERM ETAMICASTAT TREATMENT IN CONSCIOUS MICE TA-PAC20 transmitters (Data Sciences
International, St. Paul, MN) were implanted into the carotid artery of D2−/− and D2+/+ mice under isoflurane anesthesia, and blood pressures were measured on individual platforms 1 week
after the surgery [17, 18]. Etamicastat (10 mg/kg per day) or vehicle (tap water) was added in the drinking water after baseline blood pressure measurement. The mice were monitored for 5
days after starting drug treatment. Blood pressure and heart rate were recorded every 10 min throughout the study. Data were collected and stored automatically in a dedicated computer that
ran and analyzed the data (Dataquest). Thereafter, the mice were killed, kidneys were collected and the renal expression of selected G protein-coupled receptors, ROS-related enzymes, and
selected sodium transporters, exchangers, and channels were quantified, as reported [11, 12, 17,18,19,20,21,22,23]. In another study, mice implanted with TA-PAC20 transmitters were treated
with etamicastat (10 mg/kg per day in the drinking water) for 5 days and fed a normal salt (0.6% NaCl) diet. At the end of this period, the dose of etamicastat was increased to 50 mg/kg per
day for another 5 days. Blood pressure was recorded (one reading per h) on the last day on each treatment. ACUTE RENAL-SPECIFIC DOWNREGULATION OF D2R Renal cortical _Drd2_ was silenced by
the renal subcapsular infusion of _Drd2_-specific siRNA, via an osmotic minipump [13, 19, 20]. Adult male C57BL/6J mice were uninephrectomized 1 week before the implantation of the minipump.
Osmotic minipumps (ALZET® Osmotic Pump, 100 µl; flow rate 0.5 µl/h for 7 days) were filled with previously validated _Drd2_-specific siRNA (delivery rate 3 μg per day) or non-silencing
siRNA as control. The siRNAs were dissolved in an in vivo transfection reagent (TransIT® In Vivo Gene Delivery System, Mirus) under sterile conditions. The minipumps were fitted with a
polyethylene delivery tubing (Alzet #0007701) and the tip of the tubing was inserted within the subcapsular space of the remaining kidney. Etamicastat treatment (10 mg/kg per day in the
drinking water) was started immediately after pump implantation. Blood pressure was measured under pentobarbital anesthesia, as described, 7 days after pump implantation. IMMUNOBLOTTING
Whole-kidney lysates were prepared in lysis buffer supplemented with protease inhibitors, as previously reported [11, 12, 17,18,19,20,21,22,23]. Samples with equal amounts of proteins were
separated by 10% SDS-polyacrylamide gel (Bio-Rad) electrophoresis and transferred onto nitrocellulose membranes. The membranes were sequentially probed with the primary antibodies (1:5000)
at 4 °C overnight and corresponding horseradish peroxidase-conjugated secondary antibodies (1:10,000, Pierce at room temperature for 1 h). Chemiluminescence was detected using SuperSignal
West Dura Substrate (Thermo Fisher Scientific, Waltham, MA), followed by autoradiography. The band densities of the proteins of interest were quantified by the NIH Image J and normalized by
corresponding total actin bands. Alternatively, for infrared detection of protein signal, the membranes were probed with Infra-red dye 680- or 800-labeled secondary antibodies (Li-COR
Bioscience, Lincoln, NE). The band densities of the proteins of interest were quantified using the Odyssey Infrared imaging system (Li-COR) and normalized by corresponding total actin bands.
The rabbit polyclonal antibodies against DA receptors D1R (DRD1), D3R (DRD3), D4R (DRD4), and D5R (DRD5) were generated in our laboratory while rabbit polyclonal antibodies against D2R (EMD
Millipore, Billerica, MA) and actin (Sigma-Aldrich, St. Louis, MO) were purchased. We have reported the specificity of our D1R antibody [18, 20], D3R antibody [19], D4R antibody [17], D5R
antibody [21, 22], D1R antibody from Origene (Rockville, MD) [22], and D2R from EMD Millipore [13]. The sources of other antibodies used were: NHE3, NKCC2, NCC, αENaC, βENaC, γENac, generous
gifts of Dr. Mark Knepper [21]; ETBR (Alomone Labs, Jerusalem, Israel); NOX1 (NADPH Oxidase 1, Santa Cruz Biotechnologies, Dallas, TX), NOX2 (gp91 phox, Upstate Biotech/Thermo Fisher
Scientific), and HO-1 (Enzo Life Sciences, Farmingdale, NY). ASSAY OF CATECHOLAMINES Catecholamines in urine and tissue (DA and NE) were assayed by high-performance liquid chromatography
with electrochemical detection, as previously described. The lower limit of detection of DA and NE is 350 fmol [1, 15]. ASSAY OF 8-ISOPROSTANE Urinary isoprostane, a parameter of oxidative
stress, was measured by enzyme immunoassay (Cayman Chemical Company, Ann Arbor, MI) [12]. Values were corrected for urinary creatinine. STATISTICAL ANALYSIS Data are reported as mean ± SEM.
Comparisons between two groups used the Student’s _t_ test. One-way analysis of variance, followed by Holm–Sidak test, was used to assess significant differences among three or more groups.
_P_ < 0.05 was considered statistically significant. RESULTS ETAMICASTAT DECREASES BLOOD PRESSURE IN MICE WITH GERM LINE DELETION OF D2R OR RENAL-SILENCED D2R Systolic blood pressure
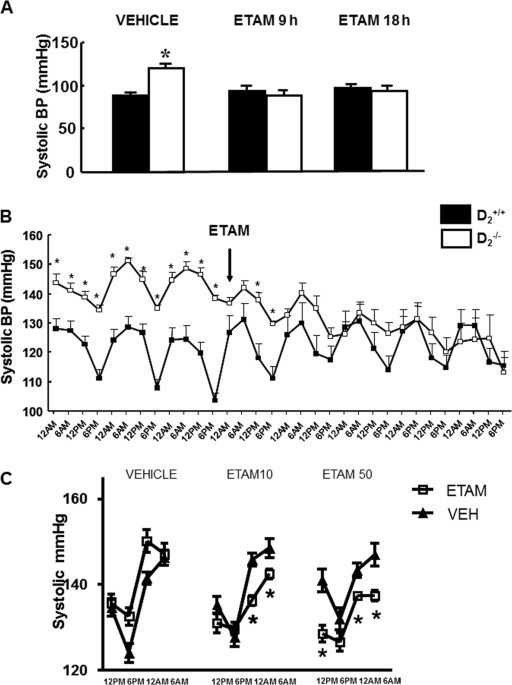
measured under anesthesia was higher in D2−/− mice than in D2+/+ littermates treated with vehicle (122 ± 1 vs. 96 ± 6 mmHg, _n_ = 4–5 per group). Systolic blood pressure also measured under
anesthesia, 9 or 18 h after gavage administration of 10 mg/kg etamicastat, was decreased in D2−/− mice to levels similar to those in D2+/+ littermates (Fig. 1a). Diastolic blood pressures,
which were increased in D2−/− mice relative to their D2+/+ littermates (91 ± 1 vs 68 ± 2 mmHg; _P_ < 0.05), were normalized at 9 h (D2−/−: 72 ± 5 mmHg; D2+/+: 72 ± 1 mmHg) and 18 h
(D2−/−: 75 ± 3 mmHg; D2+/+: 76 ± 4 mmHg) after the administration of etamicastat. Systolic blood pressure measured by telemetry in conscious mice was also higher in D2−/− than D2+/+ mice
(Fig. 1b). Administration of etamicastat (10 mg/kg per day, _n_ = 4–5 per group), added to the drinking water, also decreased systolic blood pressure in D2−/− mice but had no significant
effect in D2+/+ littermates. The decrease in systolic blood pressure was noted 24 h after starting treatment and persisted throughout the duration of the study (Fig. 1b). The decrease in
systolic blood pressure was more marked during the night when the mice are awake, feeding, and drinking water (Fig. 1c). The conscious systolic blood pressure during the day or night was
similar in mice treated for 5 days with a 10 or 50 mg/kg per day dose of etamicastat. Renal cortical-selective silencing of the D2R increased blood pressure in mice [13]. As shown in Fig.
2a, in mice implanted with an osmotic minipump for the continuous renal subcapsular infusion of D2R siRNA, renal D2R expression was decreased to about 70% as we have reported previously
[13]. These mice have increased systolic blood pressure under anesthesia. The increase in blood pressure resulting from the D2R siRNA infusion was prevented in mice treated with etamicastat
in the drinking water (10 mg/kg per day, _n_ = 5 per group) during the 7 days of D2R siRNA infusion (Fig. 2b). EFFECT OF ETAMICASTAT ON CATECHOLAMINES IN TISSUE AND URINE The cardiac NE
content was higher in vehicle-treated D2−/− than D2+/+ mice. Eighteen hours after the stomach gavage of etamicastat, cardiac NE content decreased in D2−/− mice but was minimally affected in
D2+/+ mice. Renal NE content was higher in vehicle-treated D2+/+ than in D2−/− mice. Eighteen hours after the acute administration, etamicastat had no significant effect on renal NE content
in D2+/+ or D2−/− mice. By contrast, urinary excretion of NE was similar in vehicle-treated D2+/+ and D2−/− mice; etamicastat treatment decreased NE excretion significantly in D2+/+ mice
only (Fig. 3a, _n_ = 5–8 per group). The cardiac DA content, which was lower in vehicle-treated D2−/− than D2+/+, was increased by etamicastat in D2−/− but not in D2+/+ mice. Renal DA
content was similar in vehicle-treated D2+/+ and D2−/− mice and was not significantly changed by treatment with etamicastat. However, renal DA content after etamicastat was lower in D2−/−
than D2+/+ mice. Urinary DA was similar in untreated mice of both strains but was increased by etamicastat in D2−/− but not D2+/+ mice (Fig. 3b). The DA/NE ratio was lower, although not
significantly, in tissues and urine of untreated D2−/− than in D2+/+ mice and increased by etamicastat in the heart and urine of both D2+/+ and D2−/− mice; in the kidney, etamicastat
increased the DA/NE ratio only in D2+/+ mice (Fig. 3c). EFFECT OF ETAMICASTAT ON THE RENAL EXPRESSION OF DOPAMINE AND ENDOTHELIN B RECEPTORS In D2−/− mice, relative to D2+/+ littermates, the
renal protein expressions of D3R and ETBR were increased. Etamicastat treatment did not alter the increased renal expression of D3R, but increased D1R expression, normalized ETBR
expression, and decreased D5R expression (Fig. 4a, b, _n_ = 4–5 per group). EFFECT OF ETAMICASTAT ON THE RENAL EXPRESSION OF ROS-RELATED ENZYMES In agreement with our previous report [12],
the renal expressions of NADPH oxidase isoforms NOX1 and NOX2 were increased in D2−/− mice, relative to D2+/+ littermates. Treatment with etamicastat normalized the expression of these NOX
isoforms but did not affect HO-1 expression in D2−/− mice (Fig. 5a, b, _n_ = 4–5 per group). Moreover, the urinary excretion of isoprostane, a product of the nonenzymatic oxidation of
arachidonic acid, and a marker of oxidative stress, was increased in vehicle-treated D2−/− mice and was almost completely normalized by etamicastat treatment (Fig. 6; _n_ = 4 per group).
EFFECT OF ETAMICASTAT ON THE EXPRESSION OF SELECTED RENAL SODIUM COTRANSPORTERS, EXCHANGERS, AND CHANNELS IN D2 −/− MICE The renal protein expressions of sodium hydrogen exchanger type 3
(NHE3) and sodium chloride cotransporter (NCC) were increased in D2−/− mice, relative to D2+/+ littermates. Etamicastat normalized the renal protein expression of NHE3 and decreased that of
NCC (Fig. 7a, b, _n_ = 4–5 per group). DISCUSSION The results of this study show that either the acute- or short-term administration of the peripheral DBH inhibitor, etamicastat, decreases
blood pressure in both non-anesthetized and anesthetized mice with germ line deletion of _Drd2_ or renal-selective silencing of _Drd2_ (renal subcapsular infusion of _Drd2_ siRNA in D2+/+
mice) but not in wild-type littermates or D2+/+ mice that received renal subcapsular infusion of non-silencing siRNA. The reduction in blood pressure in D2−/− mice caused by etamicastat
correlates with an increase in the DA/NE content in heart and urine (but not kidney) and D1R expression in the kidney, as well as a normalization of renal NOX isoforms (NOX1 and NOX2) and
proximal tubule NHE3 and a decrease in distal convoluted tubule NCC. These could be taken to indicate that the increase in urinary DA does not reflect total renal DA but is specific to the
nephron segment where DA is produced, i.e., renal proximal tubule. The DA produced in the renal proximal tubule is secreted in the tubular lumen affecting sodium transporters beyond the
proximal tubule [6]. The reduction in blood pressure in hypertensive D2−/− mice agrees with the reported ability of etamicastat to decrease blood pressure in hypertensive rats and humans
[3,4,5]. The hypertension in D2−/− mice is, in part, related to increased sympathetic activity proved by the ability of α1-adrenergic receptor blockade and adrenalectomy to decrease the
elevated blood pressure of D2−/− mice to the same level as that noted in D2+/+ littermates [11]. The two models we have used in this study are different in that one involves global germ line
deletion of the D2R while the other involves a 7-day renal subcapsular infusion of _Drd2_ siRNA in the remaining kidney of uninephrectomized mice that decreases renal D2R expression by
60–70%. Hemodynamic changes to compensate for the uninephrectomy may increase sympathetic nervous activity and potentiate the effect of D2R silencing. In fact, we have reported that
uninephrectomy plus silencing of D2R in the remnant kidney increases blood pressure to the same extent as that observed with global deletion of the gene; the same period (1 week) of siRNA
infusion in one kidney while the other kidney is unperturbed has no significant effect on blood pressure [13]. However, if the D2R siRNA is infused for 28 days instead of 7 days, the blood
pressure increases to the same extent as the global germ line deletion of D2R [24]. These results point to a predominant role of the kidney in the hypertension that develops in mice with
deficient D2R function. The NE content of the heart (but not kidney) was increased in vehicle-treated D2−/− mice, which may reflect the increased sympathetic activity in these mice [11, 25].
Etamicastat treatment of these D2−/− mice significantly decreased cardiac NE and increased cardiac DA. In wild-type littermates treated with etamicastat, there was a trend for NE to
decrease and DA to increase, but the effects were not statistically significant. In previous studies using etamicastat, the decrease in DBH activity and sympathetic drive was associated with
a decrease in NE in the heart of wild-type mice [1] and urine in healthy human subjects [26]. In the current study, cardiac NE content was decreased by 40% in D2−/− mice but minimally in
D2+/+, 18 h after administration of a 10 mg/kg dose of etamicastat. This modest decrease may be due to differences in sympathetic activity between D2−/− and D2+/+mice [11, 27]. By contrast,
it has been shown that, in normal mice, a 100 mg/kg dose of etamicastat decreased heart NE content by 75% [1]. Deletion of the _Dbh_ gene in mice is associated with NE deficiency. However,
the extracellular levels of DA are also decreased in the nucleus accumbens and caudate putamen but not in the prefrontal cortex and increased in adrenergic neurons, cerebellum, liver, lung,
retina, skeletal muscle, and spleen of Dbh−/− mice on mixed C57BL/6 and 129/SvEv background [28, 29]. Urine NE and DA levels, were similar in D2−/− and their wild-type littermates, in
agreement with our previous report [11] and with the failure of others to find differences in DA levels in the brain striatum of D2−/− and D2+/+ mice [30]. Basal DA efflux in the striatum
was reported to be similar in D2−/− and D2+/+ mice [30], although an increase in DA metabolites was found by others [31]. In the current study, etamicastat did not alter significantly renal
NE content. In D2+/+ mice, etamicastat decreased urinary NE. Because about 35% of urinary NE is derived from the kidney [25], this result may suggest that the inhibitory effect of
etamicastat is more pronounced or of longer duration in the heart than in the kidney. It is also possible that the failure of etamicastat to decrease NE content in the kidney may be related
to the fact that DA produced by the renal proximal tubule is not converted to NE; renal proximal tubules do not express DBH (see below). The increase in blood pressure in D2−/− mice is not
due to an increase in the activity of the renin–angiotensin system [11]. However, we have reported that the hypertension in D2−/− mice is associated with increased production of ROS,
accompanied by increased expression of NOX enzymes, results that were corroborated in the present study [12]. Treatment with etamicastat normalized the renal expression of NOX enzymes and
almost normalized the levels of ROS, as determined by urinary levels of 8-isoprostane. In D2−/− mice, treatment with spironolactone decreased blood pressure, and normalized the increased
expression of NOX1 and NOX4 but had no effect on the increased NOX2. Thus, it is possible that the increase in blood pressure in D2−/− mice drives the increase in NOX isoforms expression. In
tissues outside the central nervous system, the inhibition of DBH increases DA release [32, 33]; DA has vasorelaxant effects that cause an increase in renal blood flow and indirectly abets
the increase in sodium excretion caused by direct inhibition of renal tubular sodium transport [6]. Independent of innervation, the kidney synthesizes DA that is not metabolized to NE and
prevents the ability of moderate sodium load to increase blood pressure by inducing diuresis and natriuresis [6, 34]. Cardiac and urinary DA levels are increased in D2−/− mice after
etamicastat administration. In spite of the fact that DA produced by the kidney from L-DOPA is not metabolized to NE because DBH is not expressed in renal tubules [35], inhibition of DBH
could still increase renal DA because of blockade of NE production from renal nerves [36]. Most if not all the DA in the urine is synthesized in the kidney (specifically in the proximal
tubules) under normal conditions [6, 36, 37]. However, an increase in DA release in peripheral tissues, caused by inhibition of DBH by etamicastat may substantially increase DA in the
circulation, and thus, in the glomerular filtrate (Fig. 8). The increase in the DA/NE ratio in the urine in both groups could also reflect an increase in renal production of DA. In fact, it
has been reported that in patients with congenital DBH deficiency, not only is free plasma DA increased but also plasma DOPA that would increase the substrate for the synthesis of urinary DA
[38]. DA increases renal sodium excretion, in part, by inhibiting renal NHE3, sodium phosphate cotransporters (NaPi-IIa, NaPi-IIc), Cl-/HCO3− exchanger, sodium bicarbonate exchanger
(NBCe1), NaKATPase, NCC, ENaC, and potassium channel [6]. Treatment with etamicastat reversed the increased renal protein expressions of NHE3, NCC, and ETBR protein in D2−/− mice suggesting
that these changes were secondary to either the increased sympathetic activity or blood pressure. However, etamicastat also increased renal D1R expression, did not alter the increased D3R
expression and decreased D5R expression in D2−/− mice, indicating some specific effect on renal DA receptor expression when renal/urinary DA is increased. The human _DRD2_ gene encoding the
D2R is highly polymorphic. The presence of some single-nucleotide polymorphisms (SNPs; rs6276, rs6277, and rs1800497) in the _DRD2_ gene are associated with decreased D2R expression and
function attributable to decreased D2R messenger RNA stability and synthesis of the receptor and are associated with elevated blood pressure and hypertension [10, 39]. These SNPs are highly
prevalent with minor allele frequencies ranging from 0.24 to 0.47 [40]. From the pharmacogenomics point of view, etamicastat would be ideal to treat hypertensive patients carrying these
SNPs. In conclusion, etamicastat is effective in normalizing blood pressure in D2−/− mice, in which hypertension is caused, in part, by increased activity of the sympathetic nervous system.
In D2−/− mice, etamicastat normalized the increased renal expression on NHE3 and decreased the renal expression of NCC. Etamicastat also increased the renal expression of D1R and normalized
the increased renal expression of ETBR, decreased the renal expression of D5R without affecting the increased renal expression of D3R. Etamicastat also normalized the increased renal
expression of NOX1 and NOX2 isoenzymes. Whether or not these effects are primary or secondary to the etamicastat-induced decrease in blood pressure in D2−/− mice remains to be determined.
However, we have reported that decreasing the blood pressure of D2−/− mice with spironolactone does not normalize the increased renal NOX2 expression in D2−/− mice suggesting that the
negative regulation of NOX expression by etamicastat may be blood pressure independent. REFERENCES * Beliaev A, Learmonth DA, Soares-da-Silva P. Synthesis and biological evaluation of novel,
peripherally selective chromanyl imidazolethione-based inhibitors of dopamine beta hydroxylase. J Med Chem. 2006;49:1191–7. Article PubMed CAS Google Scholar * James PA, Oparil S,
Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E.
2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA.
2014;311:507–20. Article PubMed CAS Google Scholar * Igreja B, Pires NM, Bonifacio MJ, Loureiro AI, Fernandes-Lopes C, Wright LC, Soares-da-Silva P. Blood pressure-decreasing effect of
etamicastat alone and in combination with antihypertensive drugs in the spontaneously hypertensive rat. Hypertens Res. 2015;38:30–8. Article PubMed CAS Google Scholar * Igreja B, Wright
L, Soares-da-Silva P. Sustained high blood pressure reduction with etamicastat, a selective peripheral dopamine-β-hydroxylase inhibitor. J Am Soc Hypertens. 2016;10:207–16. Article PubMed
CAS Google Scholar * Almeida L, Nunes T, Costa R, Rocha JF, Vaz-da-Silva M, Soares-da-Silva P. Etamicastat, a novel dopamine β-hydroxylase inhibitor: tolerability, pharmacokinetics, and
pharmacodynamics in patients with hypertension. Clin Ther. 2013;35:1983–96. Article PubMed CAS Google Scholar * Armando I, Villar VA, Jose PA. Dopamine and renal function and blood
pressure regulation. Compr Physiol. 2011;1:1075–117. PubMed Google Scholar * Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep. 2008;10:268–75. Article
PubMed CAS Google Scholar * Tang L, Zheng S, Ren H, He D, Zeng C, Wang WE. Activation of angiotensin II type 1 receptors increases D4 dopamine receptor expression in rat renal proximal
tubule cells. Hypertens Res. 2017;40:652–7. Article PubMed CAS Google Scholar * Fang YJ, Thomas GN, Xu ZL, Fang JQ, Critchley JA, Tomlinson B. An affected pedigree member analysis of
linkage between the dopamine D2 receptor gene TaqI polymorphism and obesity and hypertension. Int J Cardiol. 2005;102:111–6. Article PubMed Google Scholar * Thomas GN, Tomlinson B,
Critchley JA. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension. 2000;36:177–82. Article PubMed CAS Google Scholar * Li XX, Bek
M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice.
Hypertension. 2001;38:303–8. Article PubMed CAS Google Scholar * Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species dependent hypertension in
dopamine D2 receptor-deficient mice. Hypertension. 2007;49:1–7. Article CAS Google Scholar * Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner
G, Jose PA, Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS ONE. 2012;7:e38745. Article PubMed PubMed Central CAS
Google Scholar * Ozono R, Ueda A, Oishi Y, Yano A, Kambe M, Katsuki M, Oshima T. Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J Cardiovasc
Pharmacol. 2003;42:S75–9. Article PubMed CAS Google Scholar * Soares-da-Silva P, Fernandes MH, Pinto-do-O PC. Cell inward transport of l-DOPA and 3-O-methyl-l-DOPA in rat renal tubules.
Br J Pharmacol. 1994;112:611–5. Article PubMed PubMed Central CAS Google Scholar * Soares-da-Silva P, Pestana M, Fernandes MH. Involvement of tubular sodium in the formation of dopamine
in the human renal cortex. J Am Soc Nephrol. 1993;3:1591–9. PubMed CAS Google Scholar * Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GMK, Grandy DK, Carey RM,
Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2006;47:288–95. Article PubMed CAS Google Scholar *
Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660–9.
Article PubMed PubMed Central CAS Google Scholar * Armando I, Villar VA, Jones JE, Lee H, Wang X, Asico LD, Yu P, Yang J, Escano CS Jr, Pascua-Crusan AM, Felder RA, Jose PA. Dopamine D3
receptor inhibits the ubiquitin-specific peptidase 48 to promote NHE3 degradation. FASEB J. 2014;28:1422–34. Article PubMed PubMed Central CAS Google Scholar * Villar VA, Armando I,
Sanada H, Frazer LC, Russo CM, Notario PM, Lee H, Comisky L, Russell HA, Yang Y, Jurgens JA, Jose PA, Jones JE. Novel role of sorting nexin 5 in renal D1 dopamine receptor trafficking and
function: implications for hypertension. FASEB J. 2013;27:1808–19. Article PubMed PubMed Central CAS Google Scholar * Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, Jones JE, Armando
I, Lu Q, Sibley DR, Eisner GM, Jose PA. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension. 2010;55:1431–7. Article PubMed PubMed Central CAS
Google Scholar * Wang X, Escano CS, Asico L, Jones JE, Barte A, Lau YS, Jose PA, Armando I. Upregulation of renal D5 dopamine receptor ameliorates the hypertension in D3 dopamine
receptor-deficient mice. Hypertension. 2013;62:295–301. Article PubMed PubMed Central CAS Google Scholar * Zeng C, Wang Z, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Aberrant
ETB receptor regulation of AT 1 receptors in renal proximal tubule cells of spontaneously hypertensive rats. Kidney Int. 2005;68:623–31. Article PubMed CAS Google Scholar * Konkalmatt
PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight.
2016;1:e8588 Article Google Scholar * Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–64. PubMed CAS Google Scholar * Nunes T,
Rocha JF, Vaz-da-Silva M, Falcão A, Almeida L, Soares-da-Silva P. Pharmacokinetics and tolerability of etamicastat following single and repeated administration in elderly versus young
healthy male subjects: an open-label, single-center, parallel-group study. Clin Ther. 2011;33:776–91. Article PubMed CAS Google Scholar * Pires M, Igreja B, Moura E, Wright LC, Serrao
MP, Soares-da-Silva P. Blood pressure decfease in spontanueously hypertensive rats following renal denervation or dopamine-β-hydroxylase inhibition with etamicastat. Hypertens Res.
2015;38:605–12. Article PubMed CAS Google Scholar * Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice
have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–30. PubMed CAS Google Scholar * Thomas SA, Matsumoto AM, Palmiter RD.
Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–6. Article PubMed CAS Google Scholar * Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli
E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–301. Article PubMed CAS Google Scholar * Jung MY,
Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors.
Neuroscience. 1999;91:911–24. Article PubMed CAS Google Scholar * Soares-da-Silva P. Evidence for a non-precursor dopamine pool in noradrenergic neurones of the dog mesenteric artery.
NaunynSchmiedeberg's Arch Pharmacol. 1986;333:219–23. Article CAS Google Scholar * Soares-da-Silva P. A comparison between the pattern of dopamine and noradrenaline release from
sympathetic neurones of the dog mesenteric artery. Br J Pharmacol. 1987;90:91–8. Article PubMed PubMed Central CAS Google Scholar * Hussain T, Lokhandwala MF. Renal dopamine receptors
and hypertension. Exp Biol Med. 2003;228:134–42. Article CAS Google Scholar * Hartman BK. Immunofluorescence of dopamine-β-hydroxylase. Application of improved methodology to the
localization of the peripheral and central noradrenergic nervous system. J Histochem Cytochem. 1973;21:312–32. Article PubMed CAS Google Scholar * Morgunov N, Baines AD. Renal nerves and
catecholamine excretion. Am J Physiol. 1981;240:F75–81. PubMed CAS Google Scholar * Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency
leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–54. Article PubMed PubMed Central CAS Google Scholar * Man in ‘t Veld A, Boomsma F, Lenders J, vd
Meiracker A, Julien C, Tulen J, Moleman P, Thien T, Lamberts S, Schalekamp M. Patients with congenital dopamine beta-hydroxylase deficiency. A lesson in catecholamine physiology. Am J
Hypertens. 1988;1(3 Pt 1):231–8. Article PubMed Google Scholar * Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human
dopamine receptor D2(DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–16. Article PubMed CAS Google Scholar * http://www.ncbi.nlm.nih.gov/snp.
Accessed on 7 Feb 2017. Download references ACKNOWLEDGEMENTS BIAL - Portela & Cª, S.A. supported this study. BIAL had no role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine, Division of Renal Diseases & Hypertension, The George Washington
University School of Medicine & Health Sciences, Washington, DC, 20057, USA Ines Armando, Laureano D. Asico, Xiaoyan Wang, John E. Jones, Santiago Cuevas & Pedro A. Jose * MedInUP -
Center for Drug Discovery and Innovative Medicines, University of Porto, 4200-319, Porto, Portugal Maria Paula Serrão & Patricio Soares-da-Silva * Department of Physiology and
Pharmacology, Oregon Health and Science University, Portland, OR, 97239, USA David K. Grandy * Department of Pharmacology and Physiology, The George Washington University School of Medicine
& Health Sciences, Washington, DC, 20057, USA Pedro A. Jose Authors * Ines Armando View author publications You can also search for this author inPubMed Google Scholar * Laureano D.
Asico View author publications You can also search for this author inPubMed Google Scholar * Xiaoyan Wang View author publications You can also search for this author inPubMed Google Scholar
* John E. Jones View author publications You can also search for this author inPubMed Google Scholar * Maria Paula Serrão View author publications You can also search for this author
inPubMed Google Scholar * Santiago Cuevas View author publications You can also search for this author inPubMed Google Scholar * David K. Grandy View author publications You can also search
for this author inPubMed Google Scholar * Patricio Soares-da-Silva View author publications You can also search for this author inPubMed Google Scholar * Pedro A. Jose View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ines Armando. ETHICS DECLARATIONS CONFLICT OF INTEREST P.S.S. is an employee
of BIAL - Portela & Cª, S.A. (the sponsor of the study). The remaining authors declare that they have no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Armando, I., Asico, L.D., Wang, X. _et al._ Antihypertensive effect of etamicastat in dopamine D2 receptor-deficient mice. _Hypertens Res_ 41, 489–498 (2018).
https://doi.org/10.1038/s41440-018-0041-5 Download citation * Received: 18 July 2017 * Revised: 23 October 2017 * Accepted: 30 November 2017 * Published: 13 April 2018 * Issue Date: July
2018 * DOI: https://doi.org/10.1038/s41440-018-0041-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
