Engineering redox-active electrochemically mediated carbon dioxide capture systems
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

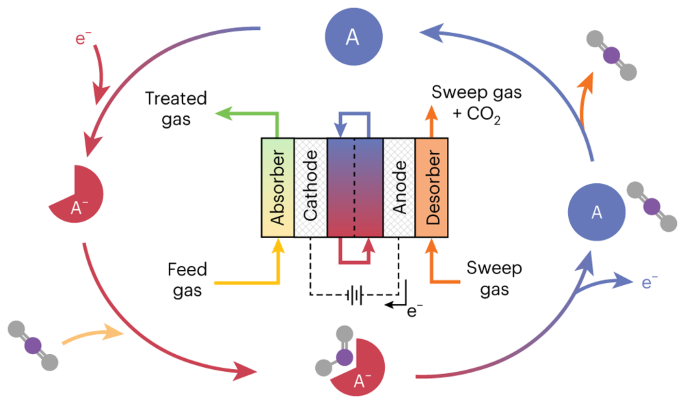
ABSTRACT With ever-increasing atmospheric carbon dioxide concentrations and commitments to limit global temperatures to less than 1.5 °C above pre-industrial levels, the need for versatile,
low-cost carbon dioxide capture technologies is paramount. Electrochemically mediated carbon dioxide separation systems promise low energetics, modular scalability and ease of
implementation, with direct integration to renewable energy for net-negative carbon dioxide operations. For these systems to be cost-competitive, key factors around their operation,
stability and scaling need to be addressed. Energy penalties associated with redox-active species transport, gas transport and bubble formation limit the volumetric productivity and scaling
potential due to their cost and footprint. Here we highlight the importance of engineering approaches towards enhancing the performance of redox-active electrochemically mediated carbon
dioxide capture systems to enable their widespread implementation. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTROCHEMICAL METHODS FOR CARBON DIOXIDE SEPARATIONS Article 08
September 2022 NON-AQUEOUS ALKOXIDE-MEDIATED ELECTROCHEMICAL CARBON CAPTURE Article 16 August 2024 CONTINUOUS DECOUPLED REDOX ELECTROCHEMICAL CO2 CAPTURE Article Open access 30 December 2024
REFERENCES * IPCC _Climate Change 2021_: _The Physical Science Basis_ (eds Masson-Delmotte, V. et al.) (Cambridge Univ. Press, 2021). * Halliday, C. & Hatton, T. A. Sorbents for the
capture of CO2 and other acid gases: a review. _Ind. Eng. Chem. Res._ 60, 9313–9346 (2021). Article CAS Google Scholar * Zhu, P. et al. Continuous carbon capture in an electrochemical
solid-electrolyte reactor - Supporting Information. _Nature_ 618, 959–966 (2023). Article CAS PubMed Google Scholar * Jiang, W. et al. Electrochemically regenerated amine for CO2 capture
driven by a proton-coupled electron transfer reaction. _Ind. Eng. Chem. Res._ 61, 13578–13588 (2022). Article CAS Google Scholar * Liu, Y., Lucas, É., Sullivan, I., Li, X. & Xiang,
C. Challenges and opportunities in continuous flow processes for electrochemically mediated carbon capture. _iScience_ 25, 105153 (2022). Article CAS PubMed PubMed Central Google Scholar
* Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. _ACS Catal._ 10, 13058–13074 (2020). Article CAS Google Scholar *
Kang, J. S., Kim, S. & Hatton, T. A. Redox-responsive sorbents and mediators for electrochemically based CO2 capture. _Curr. Opin. Green Sustain. Chem._ 31, 100504 (2021). Article CAS
Google Scholar * Gurkan, B. et al. Perspective and challenges in electrochemical approaches for reactive CO2 separations. _iScience_ 24, 103422 (2021). Article CAS PubMed PubMed Central
Google Scholar * Zito, A. M. et al. Electrochemical carbon dioxide capture and concentration. _Chem. Rev._ 123, 8069–8098 (2023). Article CAS PubMed Google Scholar * Barlow, J. M. et
al. Molecular design of redox carriers for electrochemical CO2 capture and concentration. _Chem. Soc. Rev._ 51, 8415–8433 (2022). Article CAS PubMed Google Scholar * Sullivan, B. P.,
Krist. K. & Guard, H. E. (eds) _Electrochemical and Electrocatalytic Reactions of Carbon Dioxide_ (Elsevier, 1993). * Barlow, J. M. & Yang, J. Y. Oxygen-stable electrochemical CO2
capture and concentration with quinones using alcohol additives. _J. Am. Chem. Soc._ 144, 14161–14169 (2022). Article CAS PubMed Google Scholar * Van Daele, S. et al. How flue gas
impurities affect the electrochemical reduction of CO2 to CO and formate. _Appl. Catal. B Environ._ 341, 123345 (2024). Article Google Scholar * Gurkan, B., Simeon, F. & Hatton, T. A.
Quinone reduction in ionic liquids for electrochemical CO2 separation. _ACS Sustainable Chem. Eng._ 3, 1394–1405 (2015). Article CAS Google Scholar * Diederichsen, K. M., DeWitt, S. J. A.
& Hatton, T. A. Electrochemically facilitated transport of CO2 between gas diffusion electrodes in flat and hollow fiber geometries. _ACS ES&T Eng._ 3, 1001–1012 (2023). Article
CAS Google Scholar * Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. _Energy Environ. Sci._ 12, 3530–3547 (2019). Article CAS Google Scholar
* Hemmatifar, A., Kang, J. S., Ozbek, N., Tan, K. J. & Hatton, T. A. Electrochemically mediated direct CO2 capture by a stackable bipolar cell. _ChemSusChem_ 15, e202102533 (2022).
Article CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. Electrochemically mediated gating membrane with dynamically controllable gas transport. _Sci. Adv._ 6, 22–24 (2020).
Article Google Scholar * Shaw, R. A. & Hatton, T. A. Electrochemical CO2 capture thermodynamics. _Int. J. Greenh. Gas Control_ 95, 102878 (2020). Article CAS Google Scholar *
Clarke, L. E., Leonard, M. E., Hatton, T. A. & Brushett, F. R. Thermodynamic modeling of CO2 separation systems with soluble, redox-active capture species. _Ind. Eng. Chem. Res._ 61,
10531–10546 (2022). Article CAS Google Scholar * Diederichsen, K. M. et al. Electrochemical methods for carbon dioxide separations. _Nat. Rev. Methods Prim._ 2, 2354–2374 (2022). Google
Scholar * Wang, M., Hariharan, S., Shaw, R. A. & Hatton, T. A. Energetics of electrochemically mediated amine regeneration process for flue gas CO2 capture. _Int. J. Greenh. Gas
Control_ 82, 48–58 (2019). Article CAS Google Scholar * Wang, M. & Hatton, T. A. Flue gas CO2 capture via electrochemically mediated amine regeneration: desorption unit design and
analysis. _Ind. Eng. Chem. Res._ 59, 10120–10129 (2020). Article CAS Google Scholar * Wang, M., Shaw, R., Gencer, E. & Hatton, T. A. Technoeconomic analysis of the electrochemically
mediated amine regeneration CO2 capture process. _Ind. Eng. Chem. Res._ 59, 14085–14095 (2020). Article CAS Google Scholar * Xu, Y. et al. Assessing the kinetics of quinone-CO2 adduct
formation for electrochemically mediated carbon capture. _ACS Sustain. Chem. Eng._ 11, 11333–11341 (2023). Article CAS Google Scholar * Gurkan, B., Simeon, F. & Hatton, T. A. Quinone
reduction in ionic liquids for electrochemical CO2 separation - supporting information. _ACS Sustain. Chem. Eng._ 3, 1394–1405 (2015). Article CAS Google Scholar * Wang, M. et al. Flue
gas CO2 capture via electrochemically mediated amine regeneration: system design and performance. _Appl. Energy_ 255, 113879 (2019). Article CAS Google Scholar * Diederichsen, K. M., Liu,
Y., Ozbek, N., Seo, H. & Hatton, T. A. Toward solvent-free continuous-flow electrochemically mediated carbon capture with high-concentration liquid quinone chemistry. _Joule_ 6, 221–239
(2022). Article CAS Google Scholar * Rahimi, M. et al. Carbon dioxide capture using an electrochemically driven proton concentration process. _Cell Rep. Phys. Sci._ 1, 100033 (2020).
Article Google Scholar * Rahimi, M., Catalini, G., Puccini, M. & Hatton, T. A. Bench-scale demonstration of CO2 capture with an electrochemically driven proton concentration process.
_RSC Adv._ 10, 16832–16843 (2020). Article CAS PubMed PubMed Central Google Scholar * Seo, H., Rahimi, M. & Hatton, T. A. Electrochemical carbon dioxide capture and release with a
redox-active amine. _J. Am. Chem. Soc._ 144, 2164–2170 (2022). Article CAS PubMed Google Scholar * Seo, H. & Hatton, T. A. Electrochemical direct air capture of CO2 using neutral red
as reversible redox-active material. _Nat. Commun._ 14, 313 (2023). Article CAS PubMed PubMed Central Google Scholar * Jin, S., Wu, M., Gordon, R. G., Aziz, M. J. & Kwabi, D. G. pH
swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. _Energy Environ. Sci._ 13, 3706–3722 (2020). Article CAS Google Scholar * Jin, S., Wu, M.,
Jing, Y., Gordon, R. G. & Aziz, M. J. Low energy carbon capture via electrochemically induced pH swing with electrochemical rebalancing. _Nat. Commun._ 13, 2140 (2022). Article CAS
PubMed PubMed Central Google Scholar * Leitz, F. B. & Marinčić, L. Enhanced mass transfer in electrochemical cells using turbulence promoters. _J. Appl. Electrochem._ 7, 473–484
(1977). Article CAS Google Scholar * Ke, X. et al. Rechargeable redox flow batteries: flow fields, stacks and design considerations. _Chem. Soc. Rev._ 47, 8721–8743 (2018). Article CAS
PubMed Google Scholar * Quentmeier, M., Schmid, B., Tempel, H., Kungl, H. & Eichel, R. A. Toward a stackable CO2-to-CO electrolyzer cell design─impact of media flow optimization. _ACS
Sustain. Chem. Eng._ 11, 679–688 (2023). Article CAS Google Scholar * Pérez-Gallent, E. et al. Overcoming mass transport limitations in electrochemical reactors with a pulsating flow
electrolyzer. _Ind. Eng. Chem. Res._ 59, 5648–5656 (2020). Article Google Scholar * Pei, S., You, S. & Zhang, J. Application of pulsed electrochemistry to enhanced water
decontamination. _ACS ES&T Eng._ 1, 1502–1508 (2021). Article CAS Google Scholar * Xu, Y. et al. Self-cleaning CO2 reduction systems: unsteady electrochemical forcing enables
stability. _ACS Energy Lett._ 6, 809–815 (2021). Article CAS Google Scholar * Jeon, H. S. et al. Selectivity control of Cu nanocrystals in a gas-fed flow cell through CO2 pulsed
electroreduction. _J. Am. Chem. Soc._ 143, 7578–7587 (2021). Article CAS PubMed PubMed Central Google Scholar * Compton, R. G., Eklund, J. C., Page, S. D., Mason, T. J. & Walton, D.
J. Voltammetry in the presence of ultrasound: mass transport effects. _J. Appl. Electrochem._ 26, 775–784 (1996). Article CAS Google Scholar * Xie, X. et al. Liquid-in-liquid printing of
3D and mechanically tunable conductive hydrogels. _Nat. Commun._ 14, 4289 (2023). Article CAS PubMed PubMed Central Google Scholar * Lee, Y. H. et al. Controlled synthesis of
metal-organic frameworks in scalable open-porous contactor for maximizing carbon capture efficiency. _JACS Au_ 1, 1198–1207 (2021). Article CAS PubMed PubMed Central Google Scholar *
Lee, W. H. et al. Sorbent-coated carbon fibers for direct air capture using electrically driven temperature swing adsorption. _Joule_ 7, 1241–1259 (2023). Article CAS Google Scholar *
Singh, S., Stechel, E. B. & Buttry, D. A. Transient modeling of electrochemically assisted CO2 capture and release. _J. Electroanal. Chem._ 799, 156–166 (2017). Article CAS Google
Scholar * Angulo, A., van der Linde, P., Gardeniers, H., Modestino, M. & Fernández Rivas, D. Influence of bubbles on the energy conversion efficiency of electrochemical reactors.
_Joule_ 4, 555–579 (2020). Article CAS Google Scholar * He, Y. et al. Strategies for bubble removal in electrochemical systems. _Energy Rev._ 2, 100015 (2023). Article Google Scholar *
Leonard, M. E. et al. Editors’ Choice—Flooded by success: on the role of electrode wettability in CO2 electrolyzers that generate liquid products. _J. Electrochem. Soc._ 167, 124521 (2020).
Article CAS Google Scholar * Lake, J. R., Soto, Á. M. & Varanasi, K. K. Impact of bubbles on electrochemically active surface area of microtextured gas-evolving electrodes. _Langmuir_
38, 3276–3283 (2022). Article CAS PubMed Google Scholar * Rahimi, M., Zucchelli, F., Puccini, M. & Hatton, T. A. Improved CO2 capture performance of electrochemically mediated amine
regeneration processes with ionic surfactant additives. _ACS Appl. Energy Mater._ 3, 10823–10830 (2020). Article CAS Google Scholar * Gendel, Y., Roth, H., Rommerskirchen, A., David, O.
& Wessling, M. A microtubular all CNT gas diffusion electrode. _Electrochem. Commun._ 46, 44–47 (2014). Article CAS Google Scholar * Hatton, T. A., Shaw, R. A., Wang, M. &
Voskian, S. Methods and systems for removing CO2 from a feed gas. US patent US11446604B2 (2019). * Rahimi, M. et al. An electrochemically mediated amine regeneration process with a mixed
absorbent for postcombustion CO2 capture. _Environ. Sci. Technol._ 54, 8999–9007 (2020). Article CAS PubMed Google Scholar * Wang, M., Herzog, H. J. & Hatton, T. A. CO2 capture using
electrochemically mediated amine regeneration. _Ind. Eng. Chem. Res._ 59, 7087–7096 (2020). Article CAS Google Scholar * Stern, M. C. & Hatton, T. A. Bench-scale demonstration of CO2
capture with electrochemically-mediated amine regeneration. _RSC Adv._ 4, 5906–5914 (2014). Article CAS Google Scholar * Sabatino, F. et al. Evaluation of a direct air capture process
combining wet scrubbing and bipolar membrane electrodialysis. _Ind. Eng. Chem. Res._ 59, 7007–7020 (2020). Article CAS Google Scholar * Orella, M. J., Brown, S. M., Leonard, M. E.,
Román-Leshkov, Y. & Brushett, F. R. A general technoeconomic model for evaluating emerging electrolytic processes. _Energy Technol._ 8, 1900994 (2020). Article Google Scholar * Zhang,
J. et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. _Nat. Commun._ 14, 1298 (2023). Article CAS
PubMed PubMed Central Google Scholar * Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. _Nat. Sustain._ 4, 911–919 (2021).
Article Google Scholar * Reyes, A. et al. Managing hydration at the cathode enables efficient CO2 electrolysis at commercially relevant current densities. _ACS Energy Lett._ 5, 1612–1618
(2020). Article CAS Google Scholar * Verma, S., Kim, B., Jhong, H. R. M., Ma, S. & Kenis, P. J. A. A gross-margin model for defining technoeconomic benchmarks in the electroreduction
of CO2. _ChemSusChem_ 9, 1972–1979 (2016). Article CAS PubMed Google Scholar * Kohl, A. L. & Nielsen, R. B. _Gas Purification_ (Gulf Publishing Company, 1997). * Rochelle, G. T.
Amine scrubbing for CO2 capture. _Science_ 325, 1652–1655 (2009). Article CAS PubMed Google Scholar * Merkel, T. C., Lin, H., Wei, X. & Baker, R. Power plant post-combustion carbon
dioxide capture: an opportunity for membranes. _J. Memb. Sci._ 359, 126–139 (2010). Article CAS Google Scholar * Wu, Y. et al. A submillimeter bundled microtubular flow battery cell with
ultrahigh volumetric power density. _Proc. Natl Acad. Sci. USA_ 120, e2213528120 (2023). Article CAS PubMed PubMed Central Google Scholar * Diederichsen, K. M. & Hatton, T. A.
Nondimensional analysis of a hollow fiber membrane contactor for direct air capture. _Ind. Eng. Chem. Res._ 61, 11964–11976 (2022). Article CAS Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA Michael Massen-Hane, Kyle M. Diederichsen & T. Alan
Hatton Authors * Michael Massen-Hane View author publications You can also search for this author inPubMed Google Scholar * Kyle M. Diederichsen View author publications You can also search
for this author inPubMed Google Scholar * T. Alan Hatton View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.M.-H., K.M.D. and T.A.H.
contributed to conceptualization, writing of the original draft, and review and editing of the manuscript. CORRESPONDING AUTHOR Correspondence to T. Alan Hatton. ETHICS DECLARATIONS
COMPETING INTERESTS T.A.H. is a co-founder and Scientific Advisory Board member of Verdox, Inc. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemical Engineering_ thanks Klaus Lackner and
Chang-Ha Lee for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing
agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement
and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Massen-Hane, M., Diederichsen, K.M. & Hatton, T.A. Engineering redox-active electrochemically mediated
carbon dioxide capture systems. _Nat Chem Eng_ 1, 35–44 (2024). https://doi.org/10.1038/s44286-023-00003-3 Download citation * Received: 19 August 2023 * Accepted: 20 November 2023 *
Published: 11 January 2024 * Issue Date: January 2024 * DOI: https://doi.org/10.1038/s44286-023-00003-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
