The pml1-wdr5 axis regulates h3k4me3 marks and promotes stemness of estrogen receptor-positive breast cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The alternative splicing of _PML_ precursor mRNA gives rise to various _PML_ isoforms, yet their expression profile in breast cancer cells remains uncharted. We discovered that PML1
is the most abundant isoform in all breast cancer subtypes, and its expression is associated with unfavorable prognosis in estrogen receptor-positive (ER+) breast cancers. _PML_ depletion
reduces cell proliferation, invasion, and stemness, while heterologous PML1 expression augments these processes and fuels tumor growth and resistance to fulvestrant, an FDA-approved drug for
ER+ breast cancer, in a mouse model. Moreover, PML1, rather than the well-known tumor suppressor isoform PML4, rescues the proliferation of _PML_ knockdown cells. ChIP-seq analysis reveals
significant overlap between PML-, ER-, and Myc-bound promoters, suggesting their coordinated regulation of target gene expression, including genes involved in breast cancer stem cells
(BCSCs), such as _JAG1_, _KLF4_, _YAP1_, _SNAI1_, and _MYC_. Loss of _PML_ reduces BCSC-related gene expression, and exogenous PML1 expression elevates their expression. Consistently, PML1
restores the association of PML with these promoters in _PML_-depleted cells. We identified a novel association between PML1 and WDR5, a key component of H3K4 methyltransferase (HMTs)
complexes that catalyze H3K4me1 and H3K4me3. ChIP-seq analyses showed that the loss of _PML1_ reduces H3K4me3 in numerous loci, including BCSC-associated gene promoters. Additionally, PML1,
not PML4, re-establishes the H3K4me3 mark on these promoters in _PML_-depleted cells. Significantly, PML1 is essential for recruiting WDR5, MLL1, and MLL2 to these gene promoters.
Inactivating WDR5 by knockdown or inhibitors phenocopies the effects of PML1 loss, reducing BCSC-related gene expression and tumorsphere formation and enhancing fulvestrant’s anticancer
activity. Our findings challenge the conventional understanding of PML as a tumor suppressor, redefine its role as a promoter of tumor growth in breast cancer, and offer new insights into
the unique roles of PML isoforms in breast cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS ABERRANT PROMOTER METHYLATION CONTRIBUTES TO _LRIG1_ SILENCING IN BASAL/TRIPLE-NEGATIVE BREAST
CANCER Article Open access 19 April 2022 RNA BINDING PROTEIN RBMS3 IS A COMMON EMT EFFECTOR THAT MODULATES TRIPLE-NEGATIVE BREAST CANCER PROGRESSION VIA STABILIZING PRRX1 MRNA Article 04
October 2021 THE PROAPOPTOTIC GENE INTERFERON REGULATORY FACTOR-1 MEDIATES THE ANTIPROLIFERATIVE OUTCOME OF PAIRED BOX 2 GENE AND TAMOXIFEN Article Open access 25 August 2020 INTRODUCTION
The PML protein has diverse cellular functions, including regulating cell-cycle progression, DNA damage responses, and transcription; it also plays important roles in governing immunity,
metabolism, and tumorigenesis [1,2,3,4,5,6,7]. PML protein is primarily localized in the nucleoplasm and DNA-free sub-nuclear compartments known as PML nuclear bodies (NBs) [8,9,10], which
may indirectly regulate transcription by sequestering transcription factors or serving as a platform protein for transcription factor modification [11, 12]. Several studies have suggested
that PML binds to chromatin, indicating a direct role in transcriptional regulation [6, 13, 14]. However, a systematic analysis of the global PML-bound promoters is lacking. The notion that
_PML_ is a tumor suppressor gene was mainly based on studies of the _PML4_ isoform [12, 15,16,17] and earlier clinical investigations [17]. Recent studies have revealed a more complex role
for PML in cancer. Knockdown of _PML_ inhibits the proliferation of estrogen receptor-positive (ER+) breast cancer [18] and ovarian cancer cells [19] and reduces tumor growth in mouse
xenograft models of triple-negative breast cancer (TNBC) [20,21,22] and glioblastoma [23]. Interestingly, the PML-reducing agent arsenic trioxide (ATO), an FDA-approved drug for treating
acute promyelocytic leukemia, is an effective agent in inhibiting tumor growth of glioblastoma [23, 24] and TNBCs [22]. These paradoxical findings underscore the need to revisit our
understanding of PML’s role in tumorigenesis. The _PML_ precursor mRNA undergoes alternative splicing, resulting in multiple isoforms, and the expression patterns of different _PML_ isoforms
in cancerous tissues and their specific contribution to tumorigenesis remain unknown. This study examined the expression profiles of _PML_ isoforms in normal and malignant breast cells and
tissues. We found that _PML1_ is the most abundant isoform expressed in ER+ breast tumors and cancer cell lines, with the increased _PML1_ mRNA associated with poor prognosis of luminal
breast cancer patients. Significantly, a recent clinical study revealed that the _PML_ gene is amplified in 14% of ER+ metastatic breast cancer (MBC) [25]. We also showed that the loss of
_PML_ inhibits the stemness of ER+ breast cancer cells, with elevated PML1 expression driving breast cancer stemness, tumor growth, and therapy resistance in xenograft mouse models. To
further understand the mechanism by which PML1 promotes breast tumorigenesis and stemness, we analyzed ChIP-seq data. We found that PML, Myc, and ER bind many common gene promoters,
including those encoding breast cancer stem cell (BCSC)-related genes, such as _JAG1_ [26], _KLF4_ [27], _YAP1_ [28], _SNAI1_ [29], and _MYC_ [30]. PML1 promotes the expression of both Myc
and ER target genes, thereby increasing ER+ breast cancer cell stemness. We also discovered that PML1 associates with WDR5 and regulates H3K4 tri-methylation (H3K4me3), and the inactivation
of WDR5 reduces breast cancer cell stemness and related gene expression and enhances the anticancer activity of fulvestrant. Mechanistically, PML is essential for recruiting WDR5, MLL1, and
MLL2 to the stemness gene promoters, thus regulating the H3K4me3 marks at these loci. Our findings redefine the role of PML, shifting its characterization from a tumor suppressor to a
promoter, and highlight the pivotal function of the PML1-WDR5 axis in regulating breast cancer cell stemness and drug resistance. RESULTS PML1 IS THE MOST ABUNDANT ISOFORM IN ESTROGEN
RECEPTOR-POSITIVE (ER+) BREAST TUMORS To better understand the expression patterns of _PML_ isoforms in breast cancer patients, we interrogated RNA-seq datasets from normal breast tissues
(GTEx) and breast tumors (TCGA). Our results demonstrate that the total _PML_ transcript expression is significantly elevated across all breast cancer subtypes compared to normal tissues
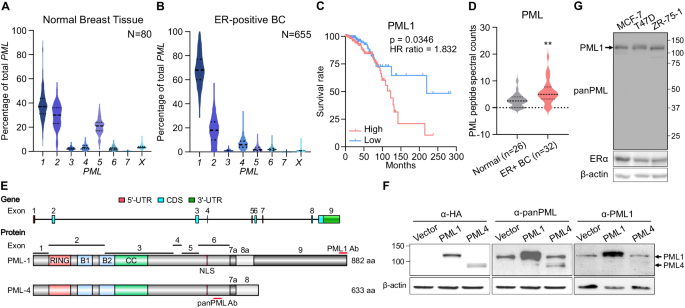
(Fig. S1). _PML1_ mRNA is the predominant isoform in normal breast tissues and ER+ breast tumors. Moreover, _PML1_ abundance shifts dramatically from ~38% in normal tissues (Fig. 1A) to ~67%
in ER+ tumors (Fig. 1B), while that of _PML2_ mRNA is expressed at a lower level than _PML1_ in both normal (~30%) and malignant breast tissues (~20%). _PML4_, which encodes an extensively
studied tumor suppressor, is expressed at a much lower level (~7%). Moreover, higher _PML1_ mRNA levels are associated with poor prognosis of ER+ breast cancer patients (Fig. 1C), but there
was no correlation between the expression of other _PML_ isoforms and prognosis (Fig. S2A). Furthermore, the total PML protein abundance is elevated in ER+ breast tumors (Fig. 1D). We also
observed a trend in which higher PML protein abundance correlates with poor prognosis (Fig. S2B). PML1 and PML4 proteins share the first 620 amino acids, with PML4 containing a 13 a.a.
unique C-terminus and PML1 possessing an additional 262 a.a (Fig. 1E). To better understand the role of PML1 in breast cancer, we generated a PML1-specific antibody. We confirmed that PML1
and PML4 proteins migrate around 130 kDa and 100 kDa, respectively (Fig. 1F) and that PML1 is the predominant isoform in ER+/HER2− breast cancer cell lines, including MCF-7, T47D, and
ZR-75-1 cells (Fig. 1G). These findings suggest that PML1 is the most abundant isoform in breast cancer, and its high expression may be a potential biomarker for poor prognosis for ER+
breast cancer. PML1 PROMOTES CANCER PHENOTYPES AND FULVESTRANT RESISTANCE Our previous study demonstrated that the ectopic overexpression of PML4 inhibits the proliferation, migration, and
invasion of MCF-7 cells [18]. We expand our studies by investigating the effects of PML on another ER+ breast cancer cell line, ZR-75-1. Our results showed that the knockdown of _PML_
reduces the proliferation (Fig. 2A), colony formation (Fig. 2D), and invasion (Fig. 2F) of MCF-7 and ZR-75-1 cells, while _PML1_ overexpression has the opposite effect (Fig. 2B, E, and G).
Furthermore, MCF-7-HA-PML1 cells, which express virally transduced HA-PML1, exhibit a significant increase in the IC50 (4.499e-008M) for fulvestrant, compared to control cells (1.046e-010M)
(Fig. 2H), indicating that higher PML1 expression promotes fulvestrant resistance. This result is consistent with a recent clinical study indicating that the _PML_ gene is amplified in 14%
of ER + MBC [25] (Fig. S3). Moreover, exogenous PML1 rescues the proliferation of _PML_ knockdown cells (Fig. 2C), but PML4 does not (Fig. S4A). Additionally, PML2 inhibits the proliferation
and breast cancer cell stemness (Fig. S4B), indicating that PML2 and PML1 have the opposite effects on breast cancer cells. These results suggest that PML isoforms play distinct roles in
breast cancer development and progression and that PML1 may play a role in fulvestrant resistance. PML1 BINDS AND POSITIVELY REGULATES STEMNESS GENE PROMOTERS AND PROMOTES BREAST CANCER
STEM-LIKE CELL (BCSC) POPULATIONS The observations that PML1 promotes fulvestrant resistance and invasion of breast cancer cells prompted us to investigate PML1’s role in cancer cell
stemness. Gene Set Enrichment Analysis (GSEA) revealed that affected genes in _PML_ knockdown microarray gene expression study are enriched for genes upregulated in the Mammary_Stem_Cell_Up
signature [31] (Fig. 3A), suggesting PML’s role in CSC regulation. Analyses of PML ChIP-seq data in MCF-7 cells revealed that PML binds to more than half of the BCSC-associated gene
promoters (Table S3). Knockdown of _PML1_ significantly reduced the expression of a subset of BCSC-related genes (Fig. 3B), while overexpression of PML1 increased their expression (Fig. 3C).
Moreover, _PML1_ knockdown reduced the frequency of BCSCs in extreme limiting dilution assays (ELDAs) (Fig. 3D) and tertiary tumorsphere-formation assays (Figs. 3F, S5), while PML1
overexpression had the opposite effect (Figs. 3E, G, and S5). FACS analyses further showed that _PML_ knockdown reduced the ALDHhigh cell population, while overexpression of PML1 increased
it (Fig. 3H, I). These results suggest that PML1 promotes the stemness of breast cancer cells. PML1 PROMOTES TUMOR GROWTH AND FULVESTRANT RESISTANCE IN A XENOGRAFT ANIMAL MODEL Next, we
determined the effects of PML1 on the tumor growth of MCF-7 cells. Our results showed that animals xenografted with MCF-7-HA-PML1 cells developed significantly larger tumors than those with
control cells (Fig. 4A, B). These findings suggest that PML1 plays a crucial role in promoting tumor growth in breast cancer. HA-PML1 protein expression is confirmed by western blots in
tumors stably express HA-PML1 (Fig. 4C), and HA-PML1-expressing tumors show elevated BCSC-related gene expression (Fig. 4D) Lastly, tumors generated with cells expressing MCF-7-HA-PML1 were
resistant to fulvestrant (Fig. 4E). These observations are consistent with the fact that 14% of ER + MBC have the _PML_ gene amplification. These findings suggest that PML1 is crucial in
promoting tumor growth and fulvestrant resistance in breast cancer. CHIP-SEQ ANALYSES REVEAL CROSSTALK BETWEEN PML1, ER, AND MYC-BOUND PROMOTERS Previous reports have shown that the Myc
transcription factor regulates the expression of a subset of stemness genes [32] and Myc interacts with PML4 [33]. Analyses of ChIP-seq data for PML, Myc, and ER revealed that most
PML-binding sites (~77%) are in promoter regions, which account for 23% of protein-coding gene promoters (Fig. 5A and E). In contrast, less than 14% of the ER-binding sites are in promoter
regions, while ~80% are in intergenic regions or introns (Fig. 5B). Interestingly, most Myc-binding sites are in intergenic regions or introns (Fig. 5C). Focusing on PML-bound promoters
(Fig. 5D), we found that PML and ER bind 1387 common promoters (Fig. 5D, E), which accounts for ~70% of ER- and ~18% of PML1-bound promoters, respectively (Figs. 5E, S6A). The top-ranked
consensus sequence among PML1 and ER commonly bound promoters is an estrogen-response element (ERE) half-site, -AGGTCA- (Fig. S6B). Myc binds ~94% of PML-bound promoters in MCF-7 cells (Fig.
5E). In fact, microarray analyses [34] suggest that affected genes in _PML_ knockdown cells are enriched in Myc-targeted genes (Fig. S6C). Furthermore, ChIP-seq analyses suggest that PML1,
Myc, and ER bind several BCSC-related gene promoters (Table S3), including _JAG1_, _KLF4_, _MYC_, _SNAI1_, and _YAP1_ (Fig. S7). Using ChIP-qPCR, we confirmed that PML binds these promoters
but not _NANOG_ (Fig. 5F) and that PML1, not PML4, binds these promoters (Fig. 5G). These analyses suggest that PML, Myc, and ER regulate gene expression in BCSCs by binding to common
promoters. Moreover, microarray gene expression analyses [34] indicate that PML target genes are enriched in estradiol-responsive genes [35] (Fig. 5H). Proximity ligation assays (PLA) showed
that endogenous PML and ER interact (Fig. S8). Furthermore, Coimmunoprecipitation demonstrated endogenous and exogenous PML1 and ER interact (Fig. 5I, J), and the recruitment of PML1 to
BCSC-related gene promoters is induced upon E2 treatment (Fig. 5K), indicating a potential role of PML1 in E2-induced ER-target gene expression. Furthermore, the knockdown of _ESR1_
significantly reduces the expression of stemness-related genes, phenocopying the effects of _PML1_ knockdown (Fig. 5L). However, the loss of _PML1_ had little or no effect on the ER binding
to these promoters (Fig. 5M), suggesting PML1 regulates ER target gene expression without affecting ER binding to the promoters. PML1 INTERACTS WITH WDR5, A CORE SUBUNIT OF THE HISTONE H3
LYSINE 4 METHYLTRANSFERASE (H3K4 HMTS) COMPLEXES To investigate the underlying mechanism of how PML1 promotes ER and Myc transcriptional activity, we utilized an _in-silico_ approach to
screen for PML1-interacting proteins, which identified several putative PML-interacting proteins involved in histone modification (Fig. 6A), including proteins involved in histone H3K4
methylation (Fig. 6B), such as WDR5 [36]. WDR5 is a core subunit of all four MLL1-4 histone methyltransferase complexes. These complexes catalyze the methylation of histone H3 lysine 4
(H3K4), with MLL1/2-containing complexes responsible for H3K4me3 and MLL3/4-containing complexes catalyzing H3K4me1 [37]. Interestingly, in silico analyses also suggest an association of PML
with MLL1. Using co-immunoprecipitation (Fig. 6C), GST pulldown assays (Fig. 6D), and PLA (Fig. S8), we showed that PML1 and WDR5 physically interact. Furthermore, we analyzed the ChIP-seq
database to examine the H3K4me3 status of PML1-, ER-, and Myc-bound promoters. Our analysis revealed that H3K4me3 marks ~88% of PML-bound promoters, and ~90% of PML, Myc, and ER
commonly-bound promoters are enriched in H3K4me3 (Fig. 6E). Specifically, several BCSC-related genes described above are enriched with the H3K4me3 mark (Fig. 6F, G). To interrogate the role
of PML1 in regulating global H3K4me3 across gene promoters, we performed _PML_ knockdown followed by ChIP-seq, which showed that PML1 regulates H3K4me3 levels at numerous gene promoters
(Fig. 7A), including gene loci associated with BCSCs (Fig. 7B). Additionally, the H3K4me3 patterns we observed on these promoters align well with publicly accessible data (Fig. S7).
Importantly, ChIP-qPCR confirmed that the loss of _PML1_ significantly reduced the H3K4me3 mark on BCSC-related gene promoters (Fig. 7C). Because PML1 and PML4 contain the WDR5-interacting
domain, we examined whether PML1 and PML4 can restore the H3K4me3 mark in _PML_ knockdown cells, and our data demonstrated that PML1, not PML4, re-establishes the H3K4me3 mark in _PML_
knockdown cells (Fig. 7D). Furthermore, the loss of _PML1_ significantly reduced the associations of WDR5 (Fig. 7E), MLL1 (Fig. 7F), and MLL2 (Fig. 7G) with stemness gene promoters. We
further investigated whether WDR5 is required for PML associations with these promoters and found that knockdown of _WDR5_ markedly reduces the expression of the BCSC-related genes (Fig. 7H)
and the H3K4me3 mark (Fig. 7I) but has little or no effect on PML1 associations with these promoters (Fig. 7J). These data suggest that PML1 promotes ER and Myc transcriptional activity
through its interaction with WDR5 and the subsequent enrichment of the H3K4me3 mark at target gene promoters. INACTIVATION OF WDR5 ENHANCES THE EFFECTIVENESS OF FULVESTRANT IN
PML1-OVEREXPRESSING CELLS The data presented above suggests that WDR5 and PML may act together to modulate the expression of stem cell-associated genes and stemness in breast cancer cells.
Our results demonstrate that the knockdown of WDR5 leads to a significant decrease in BCSCs population (Figs. 8A and S9) and inhibition of MCF-7 cell proliferation (Fig. 8B). We also found
that the knockdown of _WDR5_ significantly enhances the anti-proliferation activity of fulvestrant against PML1-overexpressing cells, reducing the IC50 from μM to nM (Fig. 8C). We next
investigated the effects of pharmacological inhibitors of WDR5, OICR-9429 and compound 16 (C16), on stemness-related gene expression, cell proliferation, and the anticancer activity of
fulvestrant. Both inhibitors disrupt the interaction between WDR5 and MLL1 by targeting their interacting sites [38, 39]. Our results demonstrated that both inhibitors effectively reduced
the population of BCSCs (Fig. 8D), inhibited the expression of stemness-related genes (Fig. 8E), and suppressed the proliferation of both control and PML1-overexpressing cells (Fig. 8F, G).
Furthermore, both inhibitors enhanced the anti-growth activity of fulvestrant (Fig. 8H). These results suggest that the PML1:WDR5 association has functional significance in regulating breast
cancer stemness and fulvestrant resistance. DISCUSSION Our study provides compelling evidence that _PML1_ promotes the proliferation, migration, and tumor growth of ER+ breast cancer cells.
We demonstrated that _PML1_ is the most abundant isoform expressed in ER+ breast tumors and plays a critical role in promoting cancer cell stemness and resistance to fulvestrant. In support
of our conclusions, a recent clinical study reported that the _PML_ gene is amplified in 14% of ER + MBC cases [25], suggesting that elevated PML1 protein promotes metastasis. These
observations affirm the notion that rather than functioning as a tumor suppressor, the _PML1_ isoform promotes breast tumorigenesis and metastasis. Moreover, we showed that PML1, not PML4,
rescues the proliferation and restores H3K4me3 of _PML_ knockdown cells. Our findings fill the knowledge gap and help to explain the conflicting data regarding the role of _PML_’s role in
tumorigenesis, which we attribute to the limited understanding of the distinct roles and abundance of different _PML_ spliced isoforms. Importantly, our study elucidates the underlying
molecular mechanism by which PML1 promotes the proliferation and stemness of ER+ breast cancer cells by regulating the stemness gene expression through the recruitment of WDR5 and
establishing the H3K4me3 mark. Despite the lack of statistical significance, we observed the trend of the effect of fulvestrant on MCF-vector tumors (Fig. 4E). The lack of statistical
significance could be due to temporal changes in tumor response. We found that tumor growth in the MCF7 vector group was significantly inhibited during the first few weeks of fulvestrant
treatment. However, some tumors showed accelerated growth in the weeks before tumor harvest, which may be due to fulvestrant treatment selecting specific cell populations within the tumor
that overcome the cytotoxic effects of fulvestrant. Additionally, sustained E2 release throughout fulvestrant treatments may reduce the efficacy of fulvestrant in inhibiting tumor growth.
Contrasting to previous reports that PML4 interacts and inhibits Myc transcription activity [40], our data showed that PML1 positively regulates _MYC_ expression and that Myc protein binds
to ~94% of PML-bound promoters, underscoring the critical role of Myc in recruiting PML to promoters and promoting cancer cell stemness. A retrospective study of ER+ breast tumors also
suggested that the _MYC_ gene amplification might contribute to endocrine therapy resistance [41,42,43]. These observations suggest that PML1 and Myc work together to promote endocrine
therapy resistance and highlight the potential of targeting the PML1-Myc axis as a therapeutic strategy for overcoming endocrine therapy resistance in ER+ breast cancer. Future research is
needed to elucidate additional mechanisms by which PML1 promotes endocrine therapy resistance, including identifying other potential players. Our study raises several important questions
that warrant further investigation. For example, it is unclear how alternative splicing controls the abundance of different _PML_ isoforms and whether this regulation is a general mechanism
that operates across different cancer types. Furthermore, our findings suggest that alternative splicing is a critical mechanism regulating tumorigenesis, highlighting the need for further
research and a potential strategy to treat breast cancer by targeting aberrant alternative splicing. These findings provide important insights into the complex regulation of _PML_ isoforms
and their role in breast cancer and lay the groundwork for future investigations into the molecular mechanisms that drive tumorigenesis. Our study highlights the importance of nucleoplasmic
PML, including chromatin-bound PML, in regulating transcription. By interrogating and combining public datasets, we identified over 12,000 PML-binding sites, primarily found in gene
promoters. Moreover, PML proteins associate with more than 70% of ER-bound promoters, and loss of PML had little or no effect on ER associations with the promoters, suggesting that PML is
recruited to chromatin by sequence-specific transcription factors, such as Myc and ER. Our data also demonstrate that PML1 promotes transcriptional activation, as evidenced by its
requirement for the enrichment of H3K4me3 (~88%) and the recruitment of WDR5, MLL1, and MLL2 on PML-bound promoters. It is worth noting that Myc binds WDR5 [44], and ER interacts with MLL2
[45], implying that WDR5 may have a broader role in regulating transcriptional activation beyond breast tumors. Previous reports suggest that WDR5 expression is a prognostic factor in breast
cancer outcomes [46] and a potential therapeutic target [47]. Recent investigations have also linked WDR5 to GBM stemness [48], indicating that it may be a potential target for treating
this type of cancer. Overall, our study highlights the importance of WDR5 in PML1-mediated gene expression to promote breast tumor growth and stemness and suggests that targeting the
PML1-WDR5 axis may be a promising therapeutic strategy for various cancers. MATERIALS AND METHODS CELL CULTURE The HEK293T and MCF-7 cell lines were procured from the American Type Culture
Collection (ATCC) and cultured on tissue culture plastic, employing Dulbecco’s Modified Eagle’s medium (DMEM) enriched with 10% fetal bovine serum (FBS) and 50 units/ml
Penicillin-Streptomycin Solution (P/S). T47D and ZR-75-1 cells (also from ATCC) were nurtured in RPMI-1640 medium supplemented with 10% FBS and 50 units/ml P/S. In the case of T47D cells, an
additional 5 μg/ml insulin was introduced into the medium. Mouse embryonic fibroblast (MEF) cells were generated in-house and cultured in DMEM supplemented with 10% FBS and 50 units/ml P/S.
All cell lines were maintained at 37 °C in a 5% CO2 incubator. Transient transfections were performed using Lipofectamine 2000 (Thermo Fisher, #11668019) following the manufacturer’s
instructions. FLOW CYTOMETRY MCF-7 and ZR-75-1 cells were dissociated, labeled with antibodies (1–2 μg per 10^6 cells for 1 h), and subsequently resuspended in 1X phosphate-buffered saline
(PBS) according to established procedures [49]. The ALDEFLUOR assay was conducted in adherence to the manufacturer’s guidelines, followed by flow cytometry using a BD Accuri C6 Plus Flow
Cytometer (BD Biosciences). Electronic gating was configured based on cells stained with the corresponding control (DEAB). Details of the antibodies used are provided in Supplementary Table
1. STATISTICAL ANALYSIS The difference in continuous measurements among groups will be determined using a t-test (two groups) assuming unequal variance or ANOVA (more than two groups)
followed by Tukey pair-wise comparison procedure. Differences between groups were considered statistically significant at values of _p_ ≤ 0.05. Data were depicted as the mean ± SD, using
***_p_ < 0.001 as significance criteria. _p_ < 0.05 and _p_ < 0.01 are designated by * and **, respectively. The likelihood ratio test and Chi-square test were used to assess the
significance. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available in the GSE255018. REFERENCES * Hsu KS, Kao HY. PML: Regulation and
multifaceted function beyond tumor suppression. Cell Biosci. 2018;8:5. Article PubMed PubMed Central Google Scholar * Bernardi R, Pandolfi PP. Structure, dynamics and functions of
promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–16. Article CAS PubMed Google Scholar * Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, et al. PML
targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–8. Article CAS PubMed PubMed Central Google Scholar * Regad T, Bellodi C, Nicotera P, Salomoni P. The
tumor suppressor Pml regulates cell fate in the developing neocortex. Nat Neurosci. 2009;12:132–40. Article CAS PubMed Google Scholar * Tessier S, Martin-Martin N, de The H, Carracedo A,
Lallemand-Breitenbach V. Promyelocytic leukemia protein, a protein at the crossroad of oxidative stress and metabolism. Antioxid Redox Signal. 2017;26:432–44. Article CAS PubMed Google
Scholar * Corpet A, Kleijwegt C, Roubille S, Juillard F, Jacquet K, Texier P, et al. PML nuclear bodies and chromatin dynamics: catch me if you can! Nucleic Acids Res. 2020;48:11890–912.
Article CAS PubMed PubMed Central Google Scholar * Patra U, Muller S. A tale of usurpation and subversion: SUMO-dependent integrity of promyelocytic leukemia nuclear bodies at the
crossroad of infection and immunity. Front Cell Dev Biol. 2021;9:696234. Article PubMed PubMed Central Google Scholar * Lallemand-Breitenbach V, de The H. PML nuclear bodies: from
architecture to function. Curr Opin Cell Biol. 2018;52:154–61. Article CAS PubMed Google Scholar * Bridger JM, Herrmann H, Munkel C, Lichter P. Identification of an interchromosomal
compartment by polymerization of nuclear-targeted vimentin. J Cell Sci. 1998;111:1241–53. Article CAS PubMed Google Scholar * Lallemand-Breitenbach V, de The H. PML nuclear bodies. Cold
Spring Harb Perspect Biol. 2010;2:a000661. Article PubMed PubMed Central Google Scholar * Zhong S, Salomoni P, Pandolfi PP. The transcriptional role of PML and the nuclear body. Nat Cell
Biol. 2000;2:E85–90. Article CAS PubMed Google Scholar * Khan MM, Nomura T, Kim H, Kaul SC, Wadhwa R, Shinagawa T, et al. Role of PML and PML-RARalpha in Mad-mediated transcriptional
repression. Mol Cell. 2001;7:1233–43. Article CAS PubMed Google Scholar * Chuang YS, Huang WH, Park SW, Persaud SD, Hung CH, Ho PC, et al. Promyelocytic leukemia protein in retinoic
acid-induced chromatin remodeling of Oct4 gene promoter. Stem Cells. 2011;29:660–9. Article CAS PubMed Google Scholar * Lo YH, Wu CC, Shih HM, Lai MZ. Selective activation of NFAT by
promyelocytic leukemia protein. Oncogene. 2008;27:3821–30. Article CAS PubMed Google Scholar * Minucci S, Nervi C, Lo Coco F, Pelicci PG. Histone deacetylases: a common molecular target
for differentiation treatment of acute myeloid leukemias? Oncogene. 2001;20:3110–5. Article CAS PubMed Google Scholar * Xu ZX, Zhao RX, Ding T, Tran TT, Zhang W, Pandolfi PP, et al.
Promyelocytic leukemia protein 4 induces apoptosis by inhibition of survivin expression. J Biol Chem. 2004;279:1838–44. Article CAS PubMed Google Scholar * Gurrieri C, Capodieci P,
Bernardi R, Scaglioni PP, Nafa K, Rush LJ, et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst. 2004;96:269–79. Article CAS PubMed
Google Scholar * Alhazmi N, Pai CP, Albaqami A, Wang H, Zhao X, Chen M, et al. The promyelocytic leukemia protein isoform PML1 is an oncoprotein and a direct target of the antioxidant
sulforaphane (SFN). Biochim Biophys Acta Mol Cell Res. 2020;1867:118707. Article CAS PubMed Google Scholar * Gentric G, Kieffer Y, Mieulet V, Goundiam O, Bonneau C, Nemati F, et al.
PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab. 2019;29:156–73.e110 Article CAS PubMed PubMed Central Google Scholar *
Martin-Martin N, Piva M, Urosevic J, Aldaz P, Sutherland JD, Fernandez-Ruiz S, et al. Stratification and therapeutic potential of PML in metastatic breast cancer. Nat Commun. 2016;7:12595.
Article CAS PubMed PubMed Central Google Scholar * Ponente M, Campanini L, Cuttano R, Piunti A, Delledonne GA, Coltella N, et al. PML promotes metastasis of triple-negative breast
cancer through transcriptional regulation of HIF1A target genes. JCI Insight. 2017;2:e87380. Article PubMed PubMed Central Google Scholar * Arreal L, Piva M, Fernandez S, Revandkar A,
Schaub-Clerigue A, Villanueva J, et al. Targeting PML in triple negative breast cancer elicits growth suppression and senescence. Cell Death Differ. 2020;27:1186–99. Article CAS PubMed
Google Scholar * Iwanami A, Gini B, Zanca C, Matsutani T, Assuncao A, Nael A, et al. PML mediates glioblastoma resistance to mammalian target of rapamycin (mTOR)-targeted therapies. Proc
Natl Acad Sci USA. 2013;110:4339–44. Article CAS PubMed PubMed Central Google Scholar * Amodeo V, A D, Betts J, Bartesaghi S, Zhang Y, Richard-Londt A, et al. A PML/Slit axis controls
physiological cell migration and cancer invasion in the CNS. Cell Rep. 2017;20:411–26. Article CAS PubMed Google Scholar * The Metastatic Breast Cancer Project (Provisional, December
2021). 2021. https://www.cbioportal.org/study/summary?id=brca_mbcproject_2022. * Simoes BM, O’Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, et al. Anti-estrogen resistance in human
breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Rep. 2015;12:1968–77. Article CAS PubMed PubMed Central Google Scholar * Yu F, Li J, Chen H, Fu J, Ray
S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–72. Article CAS PubMed
PubMed Central Google Scholar * Li YW, Xu J, Zhu GY, Huang ZJ, Lu Y, Li XQ, et al. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting
YAP/TAZ activity. Cell Death Discov. 2018;4:105. Article PubMed PubMed Central Google Scholar * Singh D, Deshmukh RK, Das A. SNAI1-mediated transcriptional regulation of
epithelial-to-mesenchymal transition genes in breast cancer stem cells. Cell Signal. 2021;87:110151. Article CAS PubMed Google Scholar * Lee KM, Giltnane JM, Balko JM, Schwarz LJ,
Guerrero-Zotano AL, Hutchinson KE, et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell
Metab. 2017;26:633–47.e637 Article CAS PubMed PubMed Central Google Scholar * Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, et al. Transcriptome analyses of mouse and
human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. Article PubMed PubMed Central Google Scholar * Gabay M, Li Y, Felsher DW.
MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4:a014241. Article PubMed PubMed Central Google Scholar * Cairo S, De Falco F,
Pizzo M, Salomoni P, Pandolfi PP, Meroni G. PML interacts with Myc, and Myc target gene expression is altered in PML-null fibroblasts. Oncogene. 2005;24:2195–203. Article CAS PubMed
Google Scholar * Cheng X, Kao HY. Microarray analysis revealing common and distinct functions of promyelocytic leukemia protein (PML) and tumor necrosis factor alpha (TNFalpha) signaling in
endothelial cells. BMC Genomics. 2012;13:453. Article CAS PubMed PubMed Central Google Scholar * Dutertre M, Gratadou L, Dardenne E, Germann S, Samaan S, Lidereau R, et al. Estrogen
regulation and physiopathologic significance of alternative promoters in breast cancer. Cancer Res. 2010;70:3760–70. Article CAS PubMed Google Scholar * Hein MY, Hubner NC, Poser I, Cox
J, Nagaraj N, Toyoda Y, et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015;163:712–23. Article CAS PubMed Google Scholar
* Sze CC, Shilatifard A. MLL3/MLL4/COMPASS family on epigenetic regulation of enhancer function and cancer. Cold Spring Harb Perspect Med. 2016;6:a026427. Article PubMed PubMed Central
Google Scholar * Grebien F, Vedadi M, Getlik M, Giambruno R, Grover A, Avellino R, et al. Pharmacological targeting of the Wdr5-MLL interaction in C/EBPalpha N-terminal leukemia. Nat Chem
Biol. 2015;11:571–8. Article CAS PubMed PubMed Central Google Scholar * Tian J, Teuscher KB, Aho ER, Alvarado JR, Mills JJ, Meyers KM, et al. Discovery and structure-based optimization
of potent and selective WD repeat domain 5 (WDR5) inhibitors containing a dihydroisoquinolinone bicyclic core. J Med Chem. 2020;63:656–75. Article CAS PubMed PubMed Central Google
Scholar * Buschbeck M, Uribesalgo I, Ledl A, Gutierrez A, Minucci S, Muller S, et al. PML4 induces differentiation by Myc destabilization. Oncogene. 2007;26:3415–22. Article CAS PubMed
Google Scholar * Yu L, Wang L, Mao C, Duraki D, Kim JE, Huang R, et al. Estrogen-independent Myc overexpression confers endocrine therapy resistance on breast cancer cells expressing
ERalphaY537S and ERalphaD538G mutations. Cancer Lett. 2019;442:373–82. Article CAS PubMed Google Scholar * Donati G, Amati B. MYC and therapy resistance in cancer: risks and
opportunities. Mol Oncol. 2022;16:3828–54. Article CAS PubMed PubMed Central Google Scholar * Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, et al. The genomic landscape of
endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–38.e426 Article CAS PubMed PubMed Central Google Scholar * Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q,
et al. Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol Cell. 2015;58:440–52. Article CAS PubMed PubMed Central Google Scholar * Mo R, Rao SM, Zhu
YJ. Identification of the MLL2 complex as a coactivator for estrogen receptor alpha. J Biol Chem. 2006;281:15714–20. Article CAS PubMed Google Scholar * Dai X, Guo W, Zhan C, Liu X, Bai
Z, Yang Y. WDR5 expression is prognostic of breast cancer outcome. PLoS ONE. 2015;10:e0124964. Article PubMed PubMed Central Google Scholar * Punzi S, Balestrieri C, D’Alesio C, Bossi D,
Dellino GI, Gatti E, et al. WDR5 inhibition halts metastasis dissemination by repressing the mesenchymal phenotype of breast cancer cells. Breast Cancer Res. 2019;21:123. Article PubMed
PubMed Central Google Scholar * Mitchell K, Sprowls SA, Arora S, Shakya S, Silver DJ, Goins CM, et al. WDR5 represents a therapeutically exploitable target for cancer stem cells in
glioblastoma. Genes Dev. 2023;37:86–102. Article CAS PubMed PubMed Central Google Scholar * Hsu KS, Zhao X, Cheng X, Guan D, Mahabeleshwar GH, Liu Y, et al. Dual regulation of Stat1 and
Stat3 by the tumor suppressor protein PML contributes to interferon alpha-mediated inhibition of angiogenesis. J Biol Chem. 2017;292:10048–60. Article CAS PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS This work was also supported by the Cytometry and Microscopy, Tissue Resources, and Athymic Animal and Preclinical Therapeutics Shared Resources
of the Case Comprehensive Cancer Center (P30CA043703). This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR002548 from the National
Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the
authors and do not necessarily represent the official views of the NIH. FUNDING This work was supported by NIH grants to R. Keri (R01CA257502, R01CA213843, R01CA206505), W. Schiemann
(R01CA236273), K. Cao (R00HD094906, R35GM150668), and H. Kao (R03CA242977). Compound C16 was a generous gift provided by Dr. Shaun Stauffer at the Center for Therapeutics Discovery Medicinal
Chemistry Core, Cleveland Clinic, Cleveland, OH 44195. Chun-Peng Pai and Han Wang are Berg fellowship recipients. Neel Agarwal and Josh Adams are recipients of the CanSur summer research
internships (NIH R25CA225461). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Biochemistry, Case Western Reserve University School of Medicine, Cleveland, OH, 44106, USA
Chun-Peng Pai, Han Wang, Neel Agarwal, Joshua A. Adams, Zhenghao Liu, Kaixiang Cao, William P. Schiemann & Hung-Ying Kao * Department of Cancer Biology, Cleveland Clinic Lerner Research
Institute, Cleveland, OH, 44195, USA Darcie D. Seachrist & Ruth A. Keri * Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, 44106, USA Darcie D.
Seachrist, Ruth A. Keri, Kaixiang Cao, William P. Schiemann & Hung-Ying Kao * Departments of Molecular Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, 44106,
USA Ruth A. Keri Authors * Chun-Peng Pai View author publications You can also search for this author inPubMed Google Scholar * Han Wang View author publications You can also search for
this author inPubMed Google Scholar * Darcie D. Seachrist View author publications You can also search for this author inPubMed Google Scholar * Neel Agarwal View author publications You can
also search for this author inPubMed Google Scholar * Joshua A. Adams View author publications You can also search for this author inPubMed Google Scholar * Zhenghao Liu View author
publications You can also search for this author inPubMed Google Scholar * Ruth A. Keri View author publications You can also search for this author inPubMed Google Scholar * Kaixiang Cao
View author publications You can also search for this author inPubMed Google Scholar * William P. Schiemann View author publications You can also search for this author inPubMed Google
Scholar * Hung-Ying Kao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS HK and CP conceived the study. CP, HW, DDS, NA, JAA, and ZL
conducted the experiments. CP, HW, DDS, RAK, KC, WPS, and HK analyzed and interpreted the data. CP and HK wrote the manuscript. All authors reviewed and edited the manuscript. CORRESPONDING
AUTHOR Correspondence to Hung-Ying Kao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL All animal studies were conducted with the
approval of the CWRU IACUC (2019-0040). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY METHODS AND FIGURE LEGENDS SUPPLEMENTARY FIGURES SUPPLEMENTARY TABLES ORIGINAL WESTERN BLOT RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pai, CP., Wang, H., Seachrist, D.D. _et al._
The PML1-WDR5 axis regulates H3K4me3 marks and promotes stemness of estrogen receptor-positive breast cancer. _Cell Death Differ_ 31, 768–778 (2024).
https://doi.org/10.1038/s41418-024-01294-6 Download citation * Received: 15 August 2023 * Revised: 27 March 2024 * Accepted: 03 April 2024 * Published: 16 April 2024 * Issue Date: June 2024
* DOI: https://doi.org/10.1038/s41418-024-01294-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
